Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Explanation is compulsory direct And not allowed Decide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis Is this a
Explanation is compulsory direct And not allowed
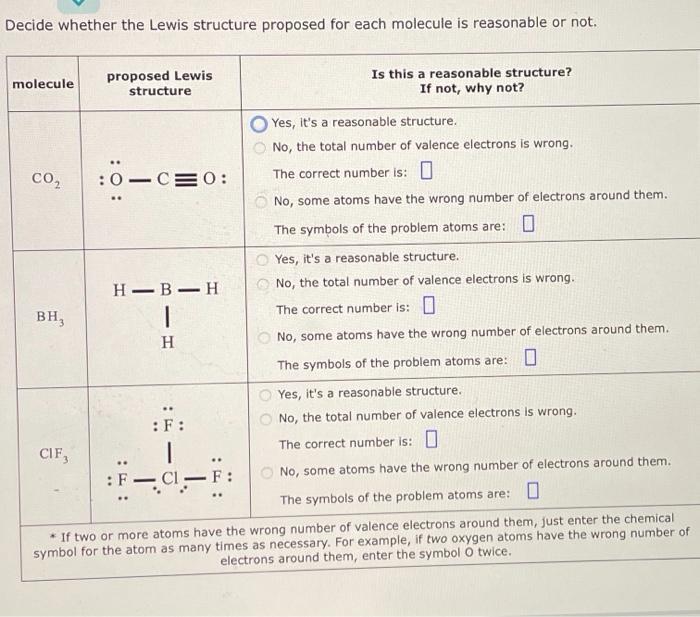
Decide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis Is this a reasonable structure? If not, why not? molecule structure Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. , :0-C= 0: The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LU Yes, it's a reasonable structure. - No, the total number of valence electrons is wrong. BH, The correct number is: O H No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LU Yes, it's a reasonable structure. ... :F: No, the total number of valence electrons is wrong. The correct number is: CIF, :F- Cl -F: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: U * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as necessary. For example, if two oxygen atoms have the wrong number of electrons around them, enter the symbol O twice. O O : :
Step by Step Solution
★★★★★
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
To write the correct lewis structures of molecules it is necessary to know Octet rule Octet rule Atoms of a chemical species will lose or gain or shar...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


