Question
The reaction of a-pinene oxide with aqueous acid includes a complex rearrangement. What would the reaction look like if a simpler molecule-cyclohexene oxide -
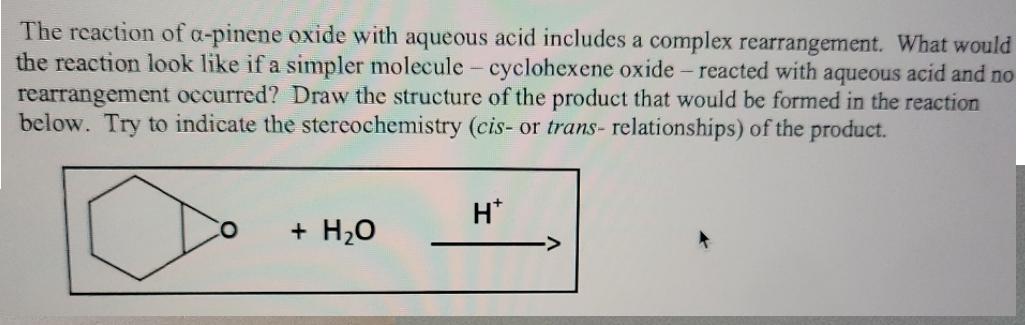
The reaction of a-pinene oxide with aqueous acid includes a complex rearrangement. What would the reaction look like if a simpler molecule-cyclohexene oxide - reacted with aqueous acid and no rearrangement occurred? Draw the structure of the product that would be formed in the reaction below. Try to indicate the stereochemistry (cis- or trans- relationships) of the product. H* O + HO
Step by Step Solution
3.30 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
Answer The reaction of cyclohexene oxide with aqueous acid inv...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Human Resource Management A Contemporary Approach
Authors: Julie Beardwell, Amanda Thompson
8th edition
129211956X, 978-1292119564
Students also viewed these Law questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



