Question
Predict the transition metal-containing products of the following reactions: a. [Mn(CO)] + H_C=CHCH2CH - CO initial product final product b. trans-Ir(CO)CI(PPh3)2 + CH31 c.
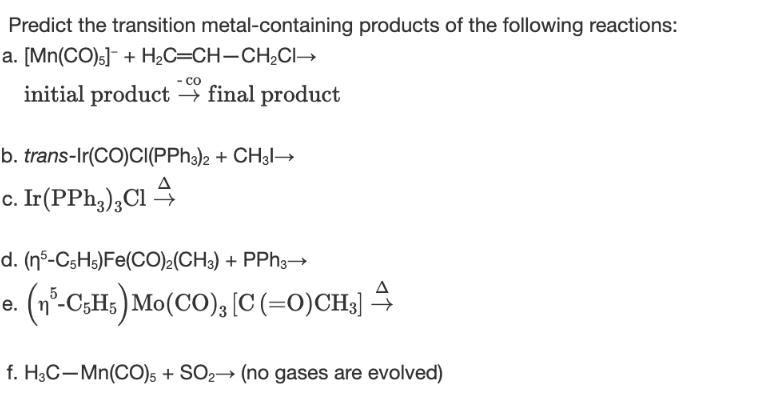
Predict the transition metal-containing products of the following reactions: a. [Mn(CO)] + H_C=CHCH2CH - CO initial product final product b. trans-Ir(CO)CI(PPh3)2 + CH31 c. Ir (PPh3)2Cl d. (n 5-C5H5) Fe(CO)2(CH3) + PPh3 e. (n-CgHs)Mo(CO),[C(=O)CH3] 4 f. H3C-Mn(CO) 5 + SO2 (no gases are evolved)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of Accounting
Authors: Needles, Powers, crosson
11th Edition
1439037744, 978-1133626985, 978-1439037744
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



