Question
Farmer Michael is developing a process in a batch reactor which can produce a new fertilizer compound. By taking secret ingredient, A, and running
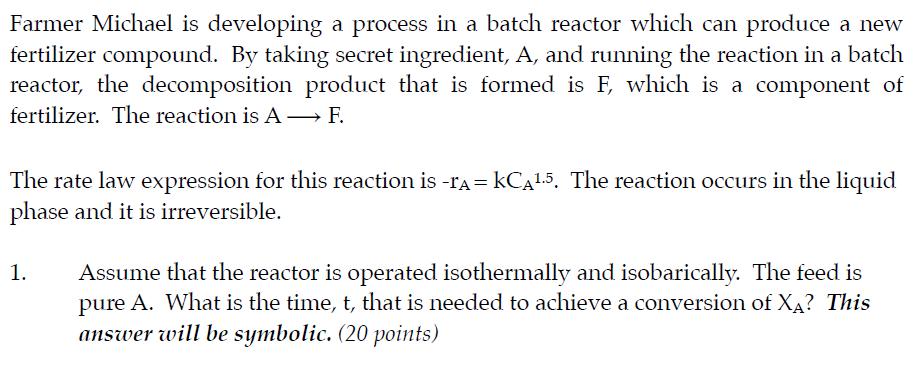
Farmer Michael is developing a process in a batch reactor which can produce a new fertilizer compound. By taking secret ingredient, A, and running the reaction in a batch reactor, the decomposition product that is formed is F, which is a component of fertilizer. The reaction is A F. The rate law expression for this reaction is -r = kCA1.5. The reaction occurs in the liquid phase and it is irreversible. 1. Assume that the reactor is operated isothermally and isobarically. The feed is pure A. What is the time, t, that is needed to achieve a conversion of XA? This answer will be symbolic. (20 points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



