Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Feed gas containing of 78.5mol2H2,21% of N2&0.5% of Ar is mixed with recycle gas and enters a reactor where 15%N2 is converted to NH3 as
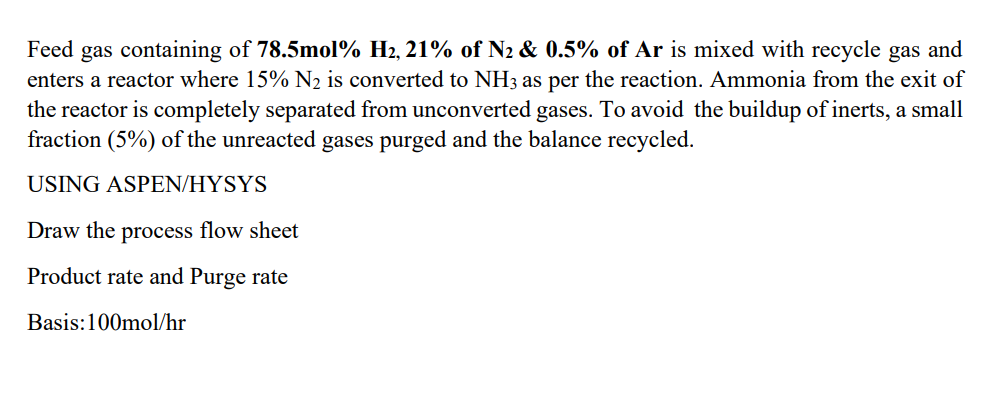 Feed gas containing of 78.5mol2H2,21% of N2&0.5% of Ar is mixed with recycle gas and enters a reactor where 15%N2 is converted to NH3 as per the reaction. Ammonia from the exit of the reactor is completely separated from unconverted gases. To avoid the buildup of inerts, a small fraction (5%) of the unreacted gases purged and the balance recycled. USING ASPEN/HYSYS Draw the process flow sheet Product rate and Purge rate Basis: 100mol/hr
Feed gas containing of 78.5mol2H2,21% of N2&0.5% of Ar is mixed with recycle gas and enters a reactor where 15%N2 is converted to NH3 as per the reaction. Ammonia from the exit of the reactor is completely separated from unconverted gases. To avoid the buildup of inerts, a small fraction (5%) of the unreacted gases purged and the balance recycled. USING ASPEN/HYSYS Draw the process flow sheet Product rate and Purge rate Basis: 100mol/hr Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


