Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Few scientists studied the kinetics of the gas phase reaction between sulfur tetrafluoride and trifluoromethyl hypofluorite in a constant volume reactor. The primary reaction is
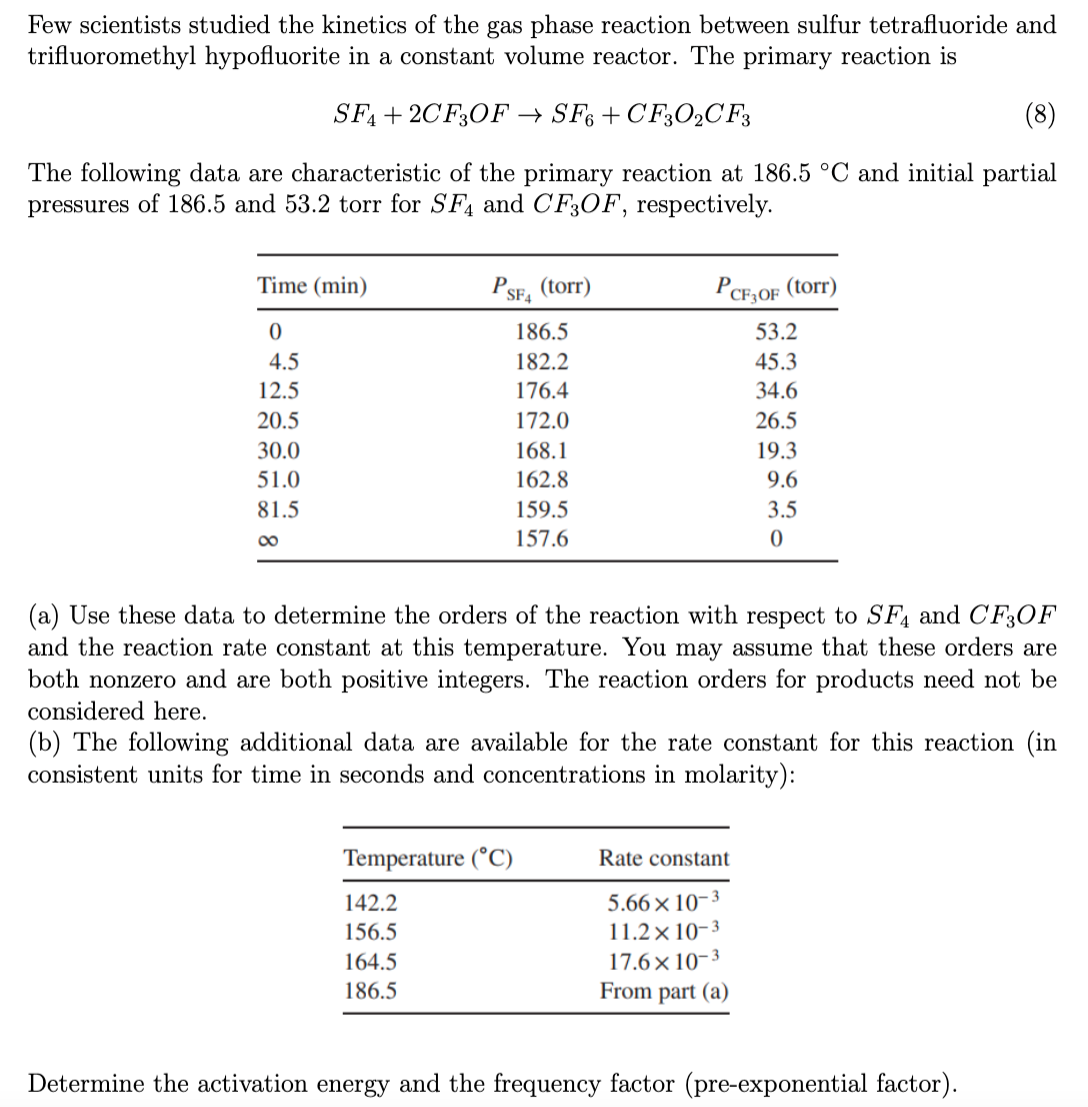 Few scientists studied the kinetics of the gas phase reaction between sulfur tetrafluoride and trifluoromethyl hypofluorite in a constant volume reactor. The primary reaction is SF4+2CF3OFSF6+CF3O2CF3 The following data are characteristic of the primary reaction at 186.5C and initial partial pressures of 186.5 and 53.2 torr for SF4 and CF3OF, respectively. (a) Use these data to determine the orders of the reaction with respect to SF4 and CF3OF and the reaction rate constant at this temperature. You may assume that these orders are both nonzero and are both positive integers. The reaction orders for products need not be considered here. (b) The following additional data are available for the rate constant for this reaction (in consistent units for time in seconds and concentrations in molarity): Determine the activation energy and the frequency factor (pre-exponential factor)
Few scientists studied the kinetics of the gas phase reaction between sulfur tetrafluoride and trifluoromethyl hypofluorite in a constant volume reactor. The primary reaction is SF4+2CF3OFSF6+CF3O2CF3 The following data are characteristic of the primary reaction at 186.5C and initial partial pressures of 186.5 and 53.2 torr for SF4 and CF3OF, respectively. (a) Use these data to determine the orders of the reaction with respect to SF4 and CF3OF and the reaction rate constant at this temperature. You may assume that these orders are both nonzero and are both positive integers. The reaction orders for products need not be considered here. (b) The following additional data are available for the rate constant for this reaction (in consistent units for time in seconds and concentrations in molarity): Determine the activation energy and the frequency factor (pre-exponential factor) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


