Answered step by step
Verified Expert Solution
Question
1 Approved Answer
FIG. 18.18 Rate of nucleation (N) of pearlite as a function of time. Eutectoid steel transformed at 680C. (Mehl, R. F., FIG. 18.19 Variation of
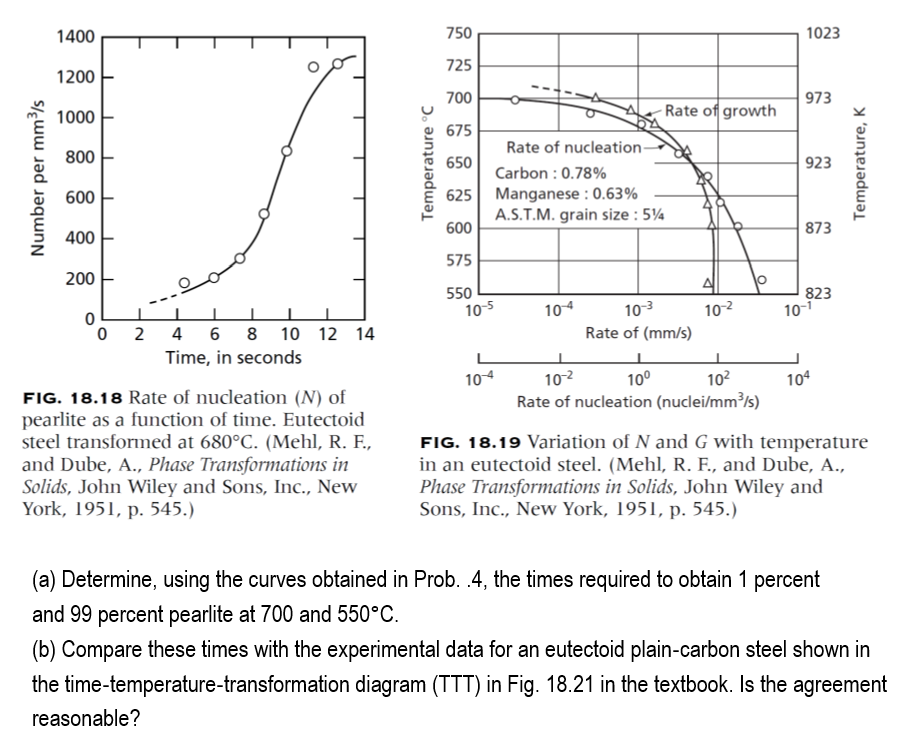 FIG. 18.18 Rate of nucleation (N) of pearlite as a function of time. Eutectoid steel transformed at 680C. (Mehl, R. F., FIG. 18.19 Variation of N and G with temperature and Dube, A., Phase Transformations in in an eutectoid steel. (Mehl, R. F., and Dube, A., Solids, John Wiley and Sons, Inc., New Phase Transformations in Solids, John Wiley and York, 1951, p. 545.) Sons, Inc., New York, 1951, p. 545.) (a) Determine, using the curves obtained in Prob. .4, the times required to obtain 1 percent and 99 percent pearlite at 700 and 550C. (b) Compare these times with the experimental data for an eutectoid plain-carbon steel shown in the time-temperature-transformation diagram (TTT) in Fig. 18.21 in the textbook. Is the agreement reasonable? FIG. 18.18 Rate of nucleation (N) of pearlite as a function of time. Eutectoid steel transformed at 680C. (Mehl, R. F., FIG. 18.19 Variation of N and G with temperature and Dube, A., Phase Transformations in in an eutectoid steel. (Mehl, R. F., and Dube, A., Solids, John Wiley and Sons, Inc., New Phase Transformations in Solids, John Wiley and York, 1951, p. 545.) Sons, Inc., New York, 1951, p. 545.) (a) Determine, using the curves obtained in Prob. .4, the times required to obtain 1 percent and 99 percent pearlite at 700 and 550C. (b) Compare these times with the experimental data for an eutectoid plain-carbon steel shown in the time-temperature-transformation diagram (TTT) in Fig. 18.21 in the textbook. Is the agreement reasonable
FIG. 18.18 Rate of nucleation (N) of pearlite as a function of time. Eutectoid steel transformed at 680C. (Mehl, R. F., FIG. 18.19 Variation of N and G with temperature and Dube, A., Phase Transformations in in an eutectoid steel. (Mehl, R. F., and Dube, A., Solids, John Wiley and Sons, Inc., New Phase Transformations in Solids, John Wiley and York, 1951, p. 545.) Sons, Inc., New York, 1951, p. 545.) (a) Determine, using the curves obtained in Prob. .4, the times required to obtain 1 percent and 99 percent pearlite at 700 and 550C. (b) Compare these times with the experimental data for an eutectoid plain-carbon steel shown in the time-temperature-transformation diagram (TTT) in Fig. 18.21 in the textbook. Is the agreement reasonable? FIG. 18.18 Rate of nucleation (N) of pearlite as a function of time. Eutectoid steel transformed at 680C. (Mehl, R. F., FIG. 18.19 Variation of N and G with temperature and Dube, A., Phase Transformations in in an eutectoid steel. (Mehl, R. F., and Dube, A., Solids, John Wiley and Sons, Inc., New Phase Transformations in Solids, John Wiley and York, 1951, p. 545.) Sons, Inc., New York, 1951, p. 545.) (a) Determine, using the curves obtained in Prob. .4, the times required to obtain 1 percent and 99 percent pearlite at 700 and 550C. (b) Compare these times with the experimental data for an eutectoid plain-carbon steel shown in the time-temperature-transformation diagram (TTT) in Fig. 18.21 in the textbook. Is the agreement reasonable Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


