Question
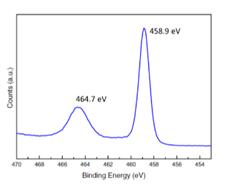
Figure 1 illustrates the X-ray Photoelectron Spectra of the Ti 2p lines for a sample of TiO 2 . The Binding Energy for the 2p3/2line
Figure 1 illustrates the X-ray Photoelectron Spectra of the Ti 2p lines for a sample of TiO2. The Binding Energy for the 2p3/2line is at 458.9 eV.
- Explain, using appropriate diagrams, how the photoelectrons are generated in X-ray Photoelectron Spectra.
- Giving your reasoning, state how the positions and relative intensities of the Ti 2p line would change if the spectra were of Ti metal rather than TiO2.
- What is the origin of the second line at 464.7 eV?
458.9 ev 464.7 ev 40 0 404 42 0 Binding Energy (ov) 456 454 (ne sunoo
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Solution 1 Xray Photoelectron SpectroscopyXPS is a photoelectric effect based quantitative spe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics The Art And Science Of Learning From Data
Authors: Alan Agresti, Christine A. Franklin
3rd Edition
9780321849281, 321755944, 321849280, 978-0321755940
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



