Answered step by step
Verified Expert Solution
Question
1 Approved Answer
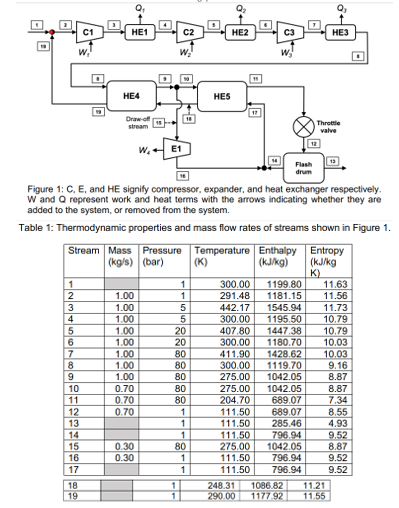
Figure 1 shows a block diagram for the Claude process that is used to liquify methane. The Claude process serves as an example to comprehensively
Figure shows a block diagram for the Claude process that is used to liquify methane. The Claude process serves as an example to comprehensively synthesize all the concepts taught from week to week of Fresh methane stream is combined with recycled methane stream and then compressed and to
bar. Intercoolers HE HE and HE after each compressor remove heat from the incoming gas. The compressed methane is then further cooled in heat exchangers HE and HE before being throttled. The flash drum operates at bar. Liquid methane exits
the flash drum in stream saturated liquid, bar A drawoff stream stream is passed through an expander E before combining with the unliquified methane stream stream saturated vapour at bar and is then recycled back streams
and The drawoff stream leaves the expander as a saturated vapour at bar. Table contains the temperature, pressure, specific enthalpy, and specific entropy of all streams. Selected mass flow rates are also provided. All compressors and expanders are adiabatic. Assume all processes to be at steady state. Neglect the kinetic and potential energy terms. a Determine whether the compressors and and the expander are reversible or irreversible.Figure : C E and HE signify compressor, expander, and heat exchanger respectively.
W and represent work and heat terms with the arrows indicating whether they are
added to the system, or removed from the system.
Table : Thermodynamic properties and mass flow rates of streams shown in Figure
b Calculate the work inputs to compressors and and the work obtained from expander in units of
c Determine the efficiency of the expander E The exhaust from the expander is saturated vapour at bar stream
d Calculate the mass flow rate of liquified methane exiting the system stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


