Answered step by step
Verified Expert Solution
Question
1 Approved Answer
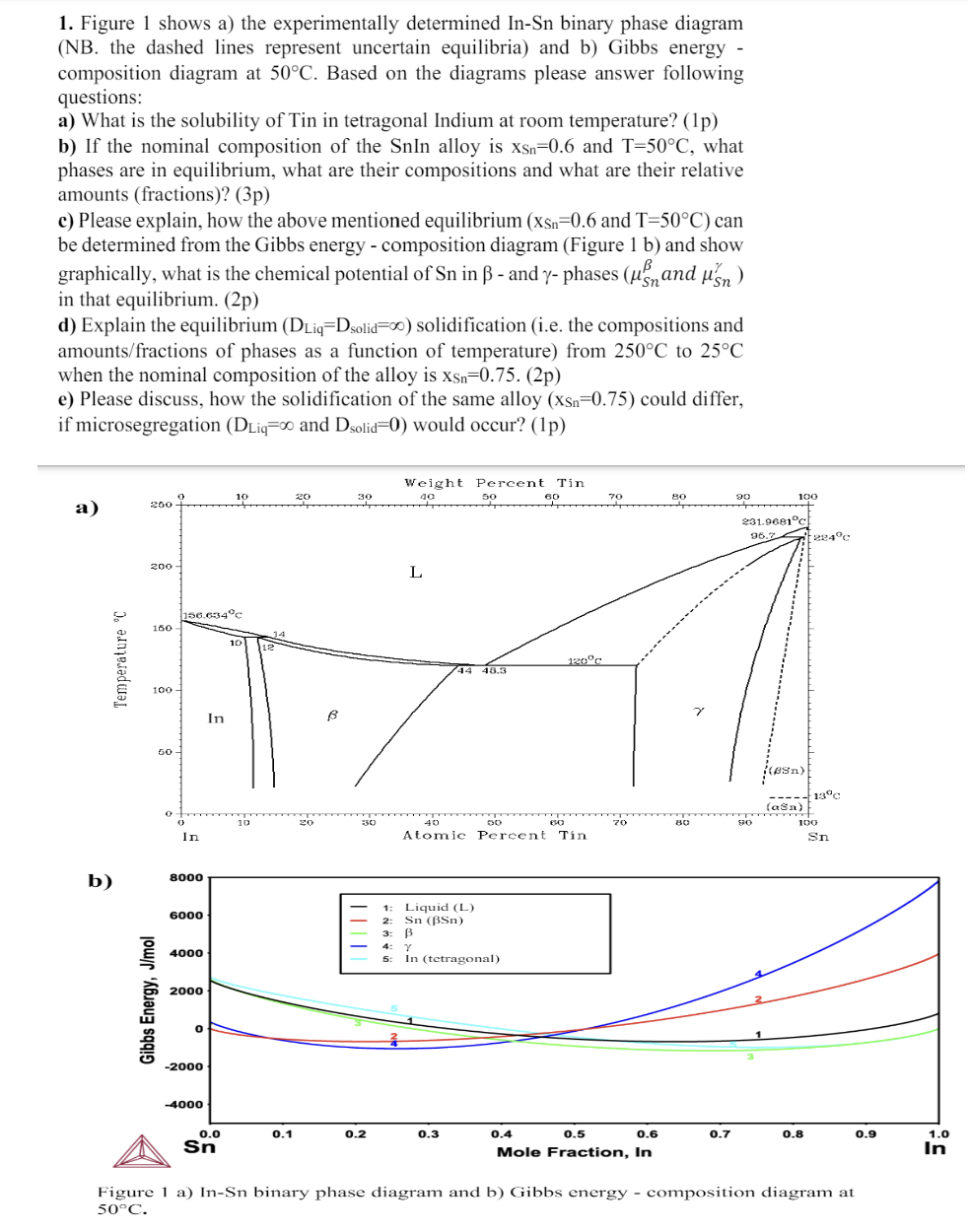
Figure 1 shows a ) the experimentally determined I n - S n binary phase diagramFigure 1 shows a ) the experimentally determined I n
Figure shows a the experimentally determined binary phase diagramFigure shows a the experimentally determined binary phase diagram
NB the dashed lines represent uncertain equilibria and b Gibbs energy
composition diagram at Based on the diagrams please answer following
questions:
a What is the solubility of Tin in tetragonal Indium at room temperature? p
b If the nominal composition of the SnIn alloy is and what
phases are in equilibrium, what are their compositions and what are their relative
amounts fractionsp
c Please explain, how the above mentioned equilibrium and : can
be determined from the Gibbs energy composition diagram Figure and show
graphically, what is the chemical potential of in and phases and
in that equilibrium. p
d Explain the equilibrium solidification ie the compositions and
amountsfractions of phases as a function of temperature from to
when the nominal composition of the alloy is p
e Please discuss, how the solidification of the same alloy could differ,
if microsegregation and would occur? p
a
Figure a InSn binary phase diagram and b Gibbs energy composition diagram at
NB the dashed lines represent uncertain equilibria and b Gibbs energy
composition diagram at Based on the diagrams please answer following
questions:
a What is the solubility of Tin in tetragonal Indium at room temperature? p
b If the nominal composition of the SnIn alloy is and what
phases are in equilibrium, what are their compositions and what are their relative
amounts fractionsp
c Please explain, how the above mentioned equilibrium and : can
be determined from the Gibbs energy composition diagram Figure and show
graphically, what is the chemical potential of in and phases and
in that equilibrium.
d Explain the equilibrium solidification ie the compositions and
amountsfractions of phases as a function of temperature from to
when the nominal composition of the alloy is p
e Please discuss, how the solidification of the same alloy : could differ,
if microsegregation and : would occur? p
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


