Answered step by step
Verified Expert Solution
Question
1 Approved Answer
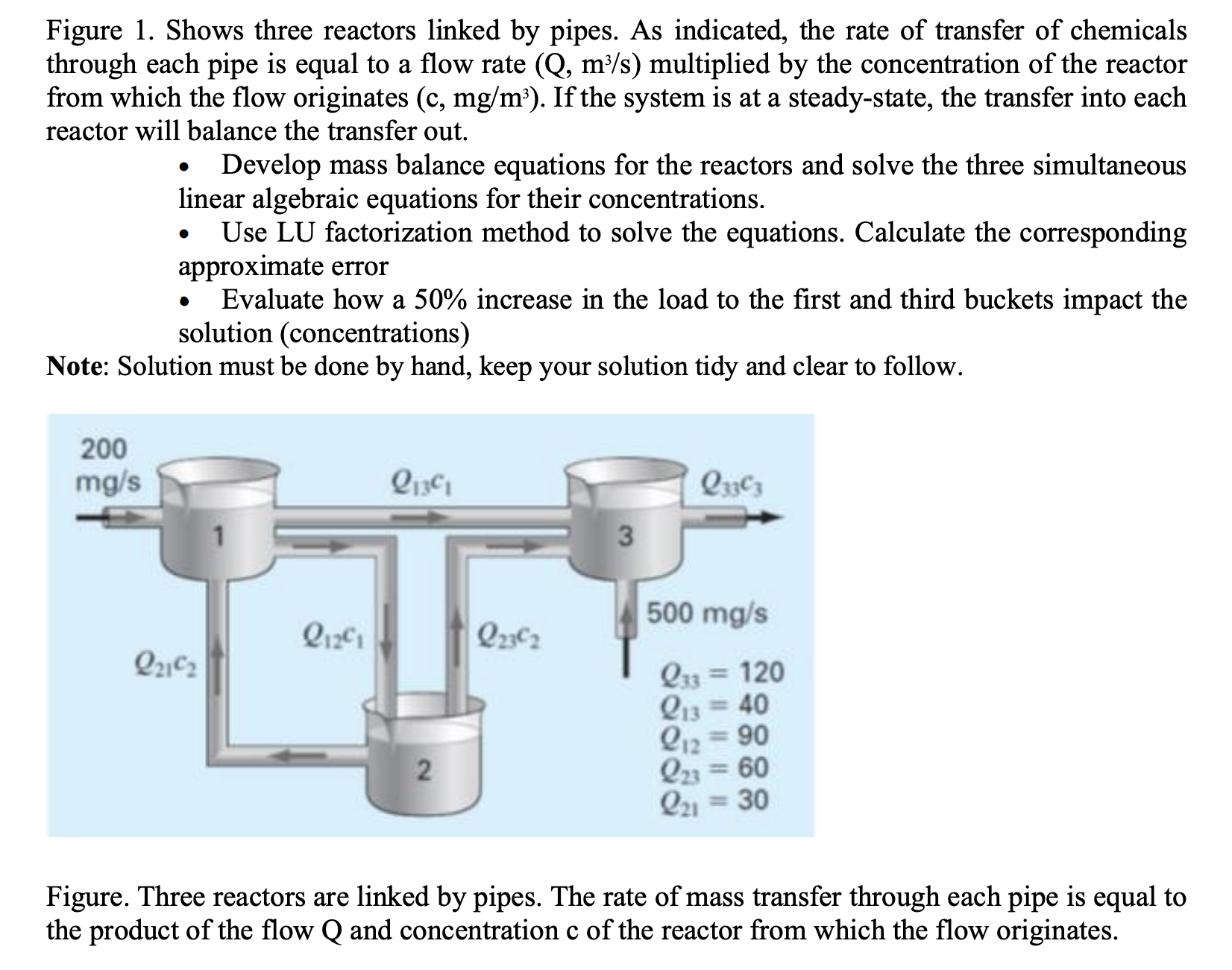
Figure 1 . Shows three reactors linked by pipes. As indicated, the rate of transfer of chemicals through each pipe is equal to a flow
Figure Shows three reactors linked by pipes. As indicated, the rate of transfer of chemicals through each pipe is equal to a flow rate multiplied by the concentration of the reactor from which the flow originates If the system is at a steadystate, the transfer into each reactor will balance the transfer out.
Develop mass balance equations for the reactors and solve the three simultaneous linear algebraic equations for their concentrations.
Use LU factorization method to solve the equations. Calculate the corresponding approximate error
Evaluate how a increase in the load to the first and third buckets impact the solution concentrations
Note: Solution must be done by hand, keep your solution tidy and clear to follow.
Figure. Three reactors are linked by pipes. The rate of mass transfer through each pipe is equal to the product of the flow and concentration of the reactor from which the flow originates.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


