Answered step by step
Verified Expert Solution
Question
1 Approved Answer
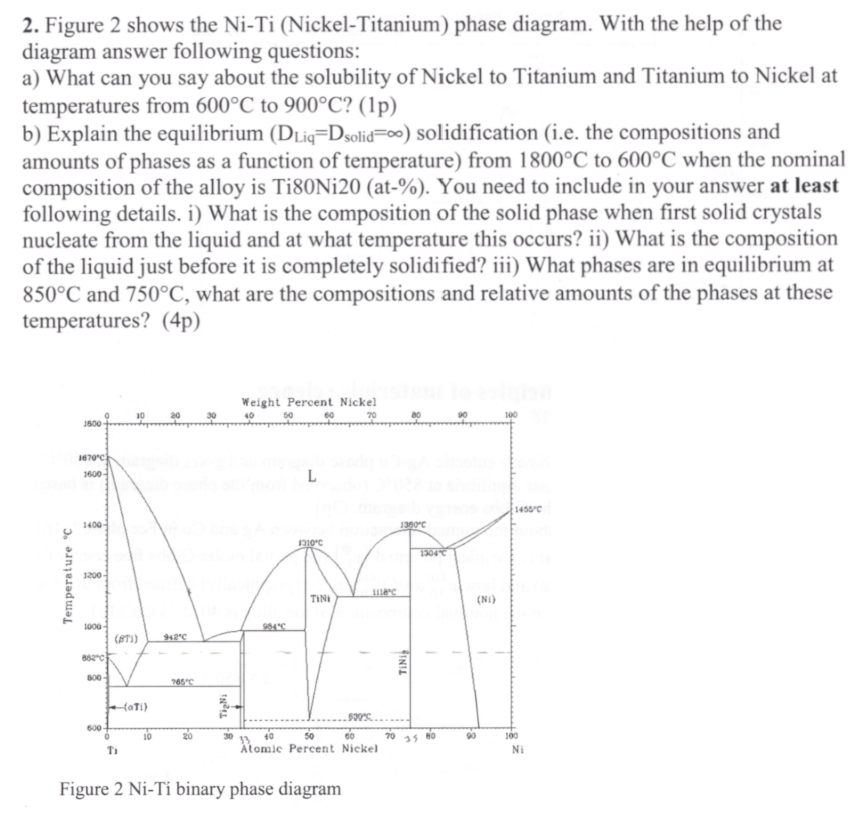
Figure 2 shows the N i - T i ( Nickel - Titanium ) phase diagram. With the help of the diagram answer following questions:
Figure shows the NickelTitanium phase diagram. With the help of the
diagram answer following questions:
a What can you say about the solubility of Nickel to Titanium and Titanium to Nickel at
temperatures from to p
b Explain the equilibrium solidification ie the compositions and
amounts of phases as a function of temperature from to when the nominal
composition of the alloy is TiNiat You need to include in your answer at least
following details. i What is the composition of the solid phase when first solid crystals
nucleate from the liquid and at what temperature this occurs? ii What is the composition
of the liquid just before it is completely solidified? iii What phases are in equilibrium at
and what are the compositions and relative amounts of the phases at these
temperatures?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


