Question
Most crystalline substances have a number of defects in which the lattice structure is broken or disrupted. In some technologies, such as in the microelectronics
Most crystalline substances have a number of defects in which the lattice structure is broken or disrupted. In some technologies, such as in the microelectronics industry, the formation of large defect-free crystal structures is vital to the per- formance of the final product. One kind of defect is an interstitial–vacancy pair in which a single atom becomes misplaced from its regular lattice site,as shown in Fig. 3.15. A very simple model of defects is the following: (1) the number of possible defects is given by the number of atoms in the crystal N; (2) each defect results in an energy penalty ε; and (3) defects are non-interacting, meaning that the presence of one defect does not affect defects adjacent to it. This latter assumption is valid at low defect density.
(a) Develop an expression for the entropy of this simple model. First, write an expression for the number of microstates as a function of the number of defects, n. Then, replace n with the total energy using the fact that E =nε. Simplify your answer using Stirling’s approximation, and rewrite it in terms of the defect density x=n/N and total number of particles N. Hint: this is a problem of combinatorics of placing defects.
(b) Find the defect density as a function of temperature. Hint: first find the temperature from the entropy.
(c) What value of defect energy ε is required for 1% of possible defects to be present at near-ambient temperature, T = 300K? For this value of ε, at what temperature will there be a 50% defect density?
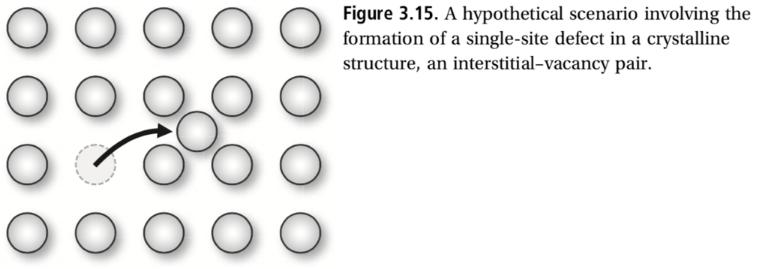
Figure 3.15. A hypothetical scenario involving the formation of a single-site defect in a crystalline structure, an interstitial-vacancy pair
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


