Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Figure shows the rates of polymerization of propylene with a single-site-type catalyst at three different temperatures (65, 80, and 95 C). The digitalized data
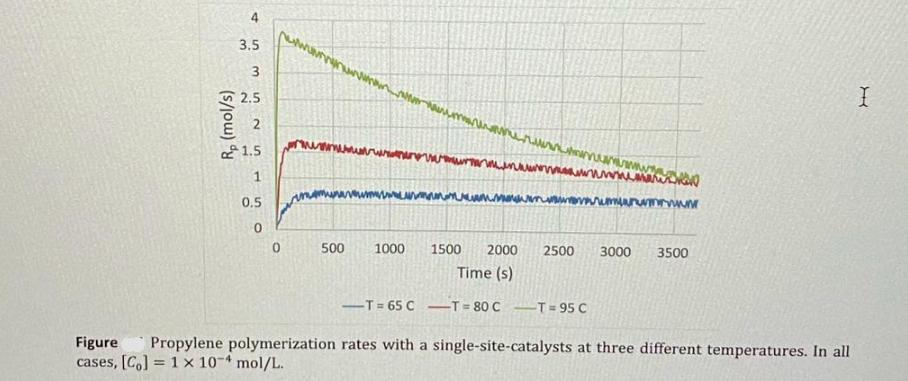
Figure shows the rates of polymerization of propylene with a single-site-type catalyst at three different temperatures (65, 80, and 95 C). The digitalized data for this MWD is available in eClass. The initial concentration of catalyst for all cases was [Co] = 1 10-4 mol/L. Estimate the activation, Ka, propagation, kp, and deactivation, kd, rate constants for this catalyst and their respective activation energies. Rp (mol/s) 4 3.5 3 2.5 2 1.5 0.5 0 www 0 www wwwwwwwwww wwwmum surten wwwwwwwwwww wa mar -T=65 C-T=80 CT=95 C www. 500 1000 1500 2000 2500 3000 3500 Time (s) Figure Propylene polymerization rates with a single-site-catalysts at three different temperatures. In all cases, [Co] 1 x 10-4 mol/L. I
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The Arrhenius equation which connects a reactions rate constant to temperature can be used to estimate the activation propagation and deactivation rate constants as well as their corresponding activat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


