Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Fill in the blanks the clear images are at the bottom the ink is black Part 1: Preparation of 0.01M standard CaCl2 solution - Weigh
Fill in the blanks 



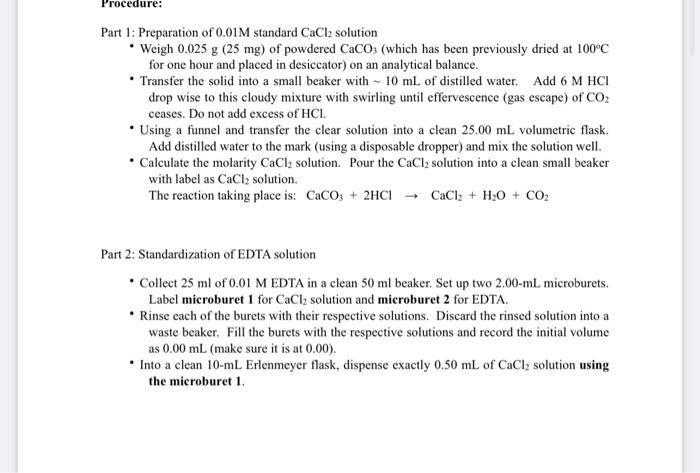



the clear images are at the bottom the ink is black
Part 1: Preparation of 0.01M standard CaCl2 solution - Weigh 0.025g (25 mg) of powdered CaCO3 (which has been previously dried at 100C for one hour and placed in desiccator) on an analytical balance. - Transfer the solid into a small beaker with 10mL of distilled water. Add 6MHCl drop wise to this cloudy mixture with swirling until effervescence (gas escape) of CO2 ceases. Do not add excess of HCl. - Using a funnel and transfer the clear solution into a clean 25.00mL volumetric flask. Add distilled water to the mark (using a disposable dropper) and mix the solution well. - Calculate the molarity CaCl2 solution. Pour the CaCl2 solution into a clean small beaker with label as CaCl2 solution. The reaction taking place is: CaCO3+2HClCaCl2+H2O+CO2 Part 2: Standardization of EDTA solution - Collect 25ml of 0.01M EDTA in a clean 50ml beaker. Set up two 2.00-mL microburets. Label microburet 1 for CaCl2 solution and microburet 2 for EDTA. - Rinse each of the burets with their respective solutions. Discard the rinsed solution into a waste beaker. Fill the burets with the respective solutions and record the initial volume as 0.00mL (make sure it is at 0.00 ). the microburet 1 . - Now, add 0.2-0.3 mL (use a disposable dropper) of ammonia buffer ( pH=10 ), followed by a very tiny amount of solid Eriochrome Black T (EBT) indicator (ask your instructor to add EBT). - The solution will assume wine red color. Record the initial volume of EDTA solution in the other microburet 2 (set it to 0.00mL ). - With constant stirring, titrate the CaCl2 (in Erlenmeyer flask) solution with EDTA (microburet 2) until the wine red color turns to blue. Next repeat the titration with the second Erlenmeyer flask (trial 2). - Calculate the molarity of EDTA solution and calculate the average from the two trials. Remember: EDTA to CaCl2 mole ratio for this titration is 1:1. Part 3: Determination of water hardness (ppm) - Empty the CaCl2 in microburet 1 and rinse it thoroughly with tap water (multiple times). Make sure microburet 1 is now clean. Now, fill the clean microburet 1 with tap water. Record its initial volume (Make sure it is 0.00mL ). - Transfer 1.50mL of tap water into a 10-mL. Erlenmeyer flask using the microburet 1 . Add 0.20.3mL (use a disposable dropper) of ammonia buffer (pH=10 ), followed by a very tiny amount of solid Eriochrome Black T (EBT) indicator (ask your instructor to add EBT). The solution will assume wine red color. - Record the initial volume of EDTA solution in the microburet 2 (make sure it is 0.00 mL ). With constant stirring, titrate the tap water in the Erlenmeyer flask with EDTA solution from the microburet 2 , until the wine red color turns to distinct blue. - Record the final volume of EDTA. Repeat the titration with the second Erlenmeyer flask (trial 2). Calculate the Ca+2 concentration in ppm. Data and Calculations: (Show all calculations) Preparation of CaCl2; Mass of CaCO3 g Mole of CaCO3 mole Mole of CaCl2= Mole of CaCO3 mole Volume of volumetric flask used L. Molarity of aqueous CaCl2 M Remember: Molarity = moles /L of solution Standardization of EDTA: Initial Volume of CaCl2(aq) Final Volume of CaCl2(aq) Volume of CaCl2(aq) used in titration (Final - Initial) Initial Volume of EDTA Final Volume of EDTA Volume of EDTA used in titration (Final - Initial) Molarity of EDTA Remember: V of CaCl2 moles of CaCl2 moles of EDTA Molarity of EDTA ppm=g Part 1: Preparation of 0.01M standard CaCl2 solution - Weigh 0.025g (25 mg) of powdered CaCO3 (which has been previously dried at 100C for one hour and placed in desiccator) on an analytical balance. - Transfer the solid into a small beaker with 10mL of distilled water. Add 6MHCl drop wise to this cloudy mixture with swirling until effervescence (gas escape) of CO2 ceases. Do not add excess of HCl. - Using a funnel and transfer the clear solution into a clean 25.00mL volumetric flask. Add distilled water to the mark (using a disposable dropper) and mix the solution well. - Calculate the molarity CaCl2 solution. Pour the CaCl2 solution into a clean small beaker with label as CaCl2 solution. The reaction taking place is: CaCO3+2HClCaCl2+H2O+CO2 Part 2: Standardization of EDTA solution - Collect 25ml of 0.01M EDTA in a clean 50ml beaker. Set up two 2.00-mL microburets. Label microburet 1 for CaCl2 solution and microburet 2 for EDTA. - Rinse each of the burets with their respective solutions. Discard the rinsed solution into a waste beaker. Fill the burets with the respective solutions and record the initial volume as 0.00mL (make sure it is at 0.00 ). the microburet 1 . - Now, add 0.2-0.3 mL (use a disposable dropper) of ammonia buffer ( pH=10 ), followed by a very tiny amount of solid Eriochrome Black T (EBT) indicator (ask your instructor to add EBT). - The solution will assume wine red color. Record the initial volume of EDTA solution in the other microburet 2 (set it to 0.00mL ). - With constant stirring, titrate the CaCl2 (in Erlenmeyer flask) solution with EDTA (microburet 2) until the wine red color turns to blue. Next repeat the titration with the second Erlenmeyer flask (trial 2). - Calculate the molarity of EDTA solution and calculate the average from the two trials. Remember: EDTA to CaCl2 mole ratio for this titration is 1:1. Part 3: Determination of water hardness (ppm) - Empty the CaCl2 in microburet 1 and rinse it thoroughly with tap water (multiple times). Make sure microburet 1 is now clean. Now, fill the clean microburet 1 with tap water. Record its initial volume (Make sure it is 0.00mL ). - Transfer 1.50mL of tap water into a 10-mL. Erlenmeyer flask using the microburet 1 . Add 0.20.3mL (use a disposable dropper) of ammonia buffer (pH=10 ), followed by a very tiny amount of solid Eriochrome Black T (EBT) indicator (ask your instructor to add EBT). The solution will assume wine red color. - Record the initial volume of EDTA solution in the microburet 2 (make sure it is 0.00 mL ). With constant stirring, titrate the tap water in the Erlenmeyer flask with EDTA solution from the microburet 2 , until the wine red color turns to distinct blue. - Record the final volume of EDTA. Repeat the titration with the second Erlenmeyer flask (trial 2). Calculate the Ca+2 concentration in ppm. Data and Calculations: (Show all calculations) Preparation of CaCl2; Mass of CaCO3 g Mole of CaCO3 mole Mole of CaCl2= Mole of CaCO3 mole Volume of volumetric flask used L. Molarity of aqueous CaCl2 M Remember: Molarity = moles /L of solution Standardization of EDTA: Initial Volume of CaCl2(aq) Final Volume of CaCl2(aq) Volume of CaCl2(aq) used in titration (Final - Initial) Initial Volume of EDTA Final Volume of EDTA Volume of EDTA used in titration (Final - Initial) Molarity of EDTA Remember: V of CaCl2 moles of CaCl2 moles of EDTA Molarity of EDTA ppm=g Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


