Question
Find K, at 600 K for NO4(g) = 2NO(g) (a) using the approximation that AH is independent of T; (b) using the approximation that
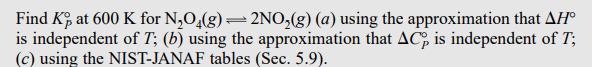

Find K, at 600 K for NO4(g) = 2NO(g) (a) using the approximation that AH is independent of T; (b) using the approximation that AC, is independent of T; (c) using the NIST-JANAF tables (Sec. 5.9). (b) If AC is assumed independent of T, then Eq. (5.19) gives AH(T) = AH (T) + AC (T) (T - T). Substitution of this equation into (6.37) gives an equation for In [K(T)/K(T)] that involves AC (T) as well as AH(T); see Prob. 6.17. Appendix data give ACP.298 = -2.88 J/mol-K, and substitution in the equation of Prob. 6.17 gives (Prob. 6.17) KP.600 1.52 x 104. =
Step by Step Solution
There are 3 Steps involved in it
Step: 1
ln K H 0 AH R 8314 Jmol k ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry Principles And Modern Applications
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
11th Edition
0132931281, 978-0132931281
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



