Question
Find the flame temperature of the products of combustion of hydrogen and oxygen whose composition and average specific heats are given in the table
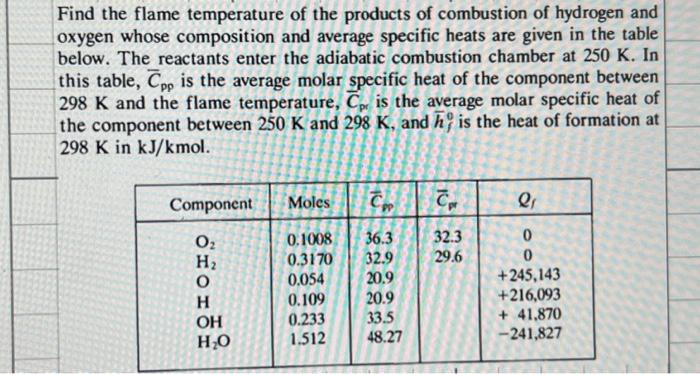
Find the flame temperature of the products of combustion of hydrogen and oxygen whose composition and average specific heats are given in the table below. The reactants enter the adiabatic combustion chamber at 250 K. In this table, Cpp is the average molar specific heat of the component between 298 K and the flame temperature, Cp is the average molar specific heat of the component between 250 K and 298 K, and h, is the heat of formation at 298 K in kJ/kmol. Component 0 OH HO Moles C 0.1008 0.3170 0.054 0.109 0.233 1.512 36.3 32.9 20.9 20.9 33.5 48.27 Cpr 32.3 29.6 a Q 0 0 +245,143 +216,093 +41,870 -241,827
Step by Step Solution
3.57 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



