Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Find the initial concentration of the [I - ] and[H 2 O 2 ] with the equationM 1 V 1 =M 2 V 2 .
Find the initial concentration of the [I-] and[H2O2] with the equationM1V1=M2V2. Treat each solution as being diluted from its initial volume and concentration to the total solution volume of 10mL.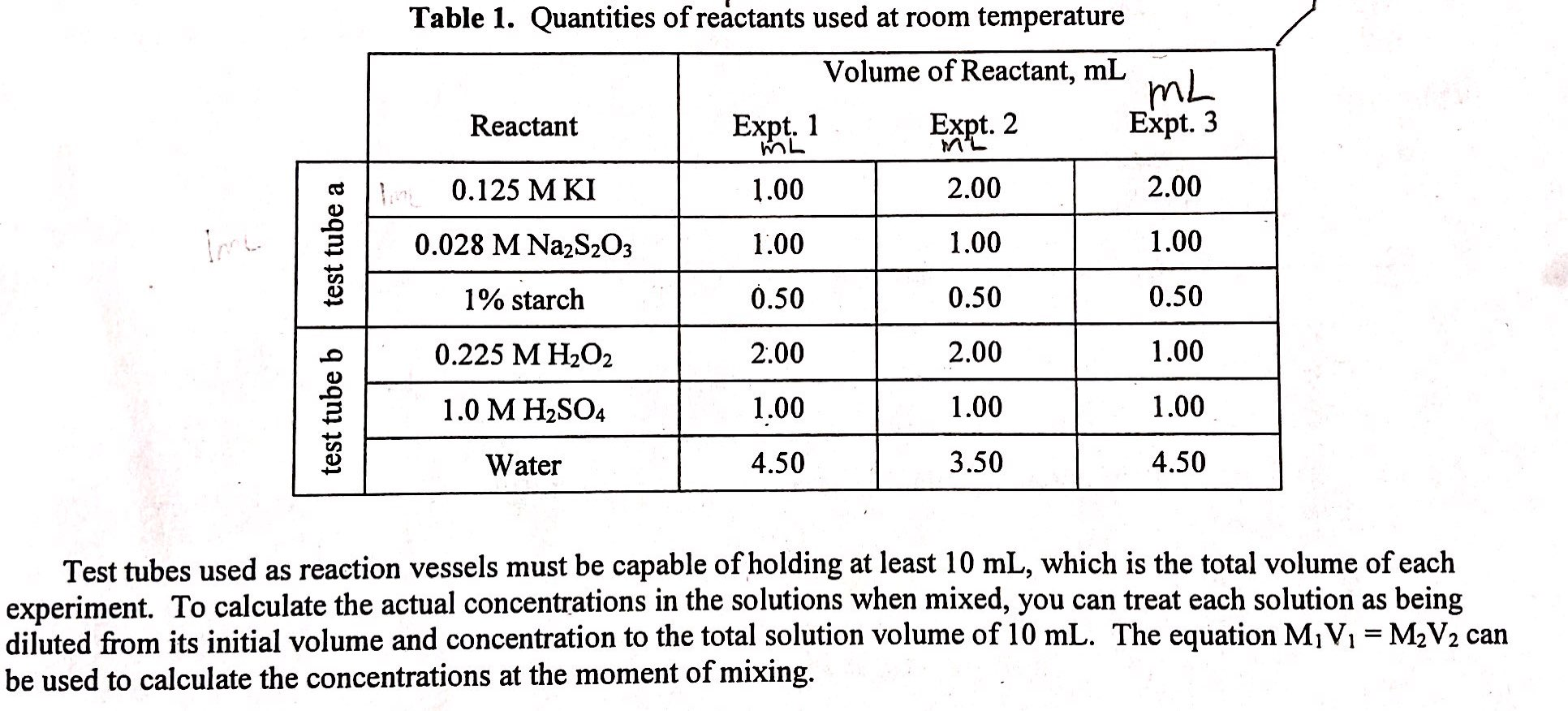
test tube a test tube b Table 1. Quantities of reactants used at room temperature Volume of Reactant, mL Reactant 0.125 M KI 0.028 M Na2SO3 1% starch 0.225 M H2O2 1.0 M HSO4 Water Expt. 1 ML 1.00 1.00 0.50 2.00 1.00 4.50 Expt. 2 ML 2.00 1.00 0.50 2.00 1.00 3.50 mL Expt. 3 2.00 1.00 0.50 1.00 1.00 4.50 Test tubes used as reaction vessels must be capable of holding at least 10 mL, which is the total volume of each experiment. To calculate the actual concentrations in the solutions when mixed, you can treat each solution as being diluted from its initial volume and concentration to the total solution volume of 10 mL. The equation M V = MV can be used to calculate the concentrations at the moment of mixing.
Step by Step Solution
★★★★★
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
a Concentration of I According to que at the time of mixing final volume of solution V2 10 mL final ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


