Find the value of (N) by therotical calculation, without using excel
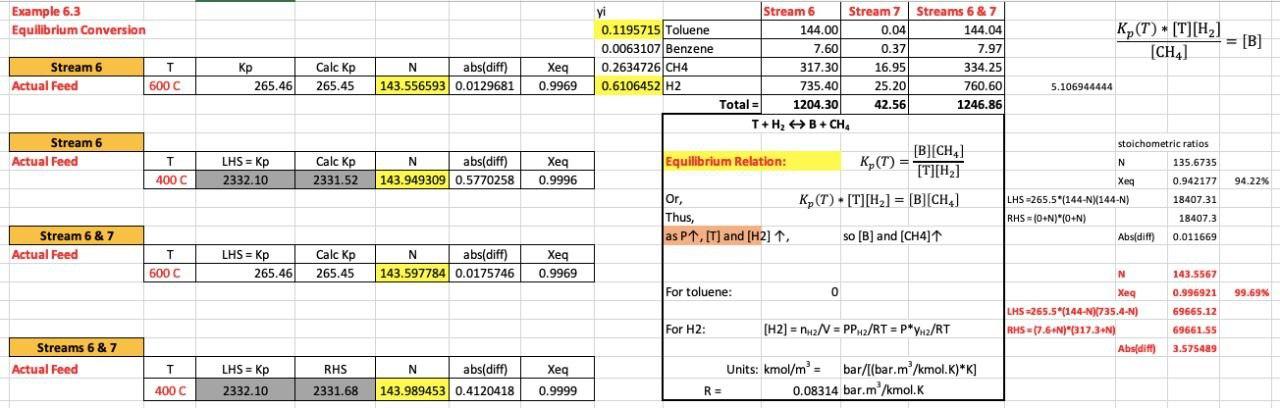
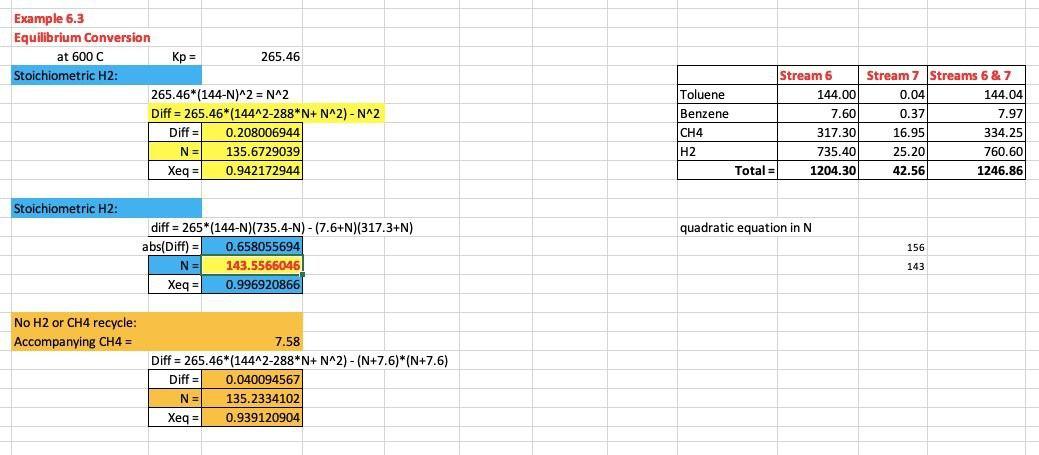
Example 6.3 Equilibrium Conversion Streams 6 & 7 144.04 7.97 K(T) * (T)[H2) = [B] [CH4] Stream 6 Actual Feed T 600 C 265.46 Calc Kp 265.45 N abs(diff) 143.556593 0.0129681 Xeg 0.9969 yi Stream 6 Stream 7 0.1195715 Toluene 144.00 0.04 0.0063107 Benzene 7.60 0.37 0.2634726|CH4 317.30 16.95 0.6106452 H2 735.40 25.20 Total = 1 1204.30 42.56 + H, B + CH. 334.25 760.60 1246.86 5.106944444 stoichometric ratios Stream 6 Actual Feed N T 400C LHS = Kp 2332.10 Equilibrium Relation: Calc Kp 2331.52 N abs(diff) 143.949309 0.5770258 Xeg 0.9996 [B][CH] K,(T) = [T][H] K(T) - [T][H2) = [B] [CH] Xeg 94.22% 135.6735 0.942177 18407.31 18407,3 Or, , Thus, as P1, [T] and [H2] 1. LHS =265.5*(144-NX144-N) RHS = 10+N)"0+N) Abs(dift) = SO [B] and (CH41 0.011669 Stream 6 & 7 Actual Feed T 600C LHS = Rp 265.46 Calc Kp 265.45 N abs(diff) 143.597784 0.0175746 Xeg 0.9969 N 143.5567 For toluene: 0 99.69% 0.996921 69665.12 Xeg LHS-265.5*(144-N)[735.4-N) -(-NN RHS 7.6.N)"317.3N) Abs(dift) For H2: [H2) = n2N = PP.2/RT =P*Yu2/RT 69661.55 3.575489 Streams 6 & 7 Actual Feed T LHS = Kp RHS N abs(diff) 143.989453 0.4120418 Xeq 0.9999 Units: kmol/m' bar/[(bar.m/kmol.K)*K] R = 0.08314 bar.m/kmol.K 400C 2332.10 2331.68 Example 6.3 Equilibrium Conversion at 600 C Kp = 265.46 Stoichiometric H2: 265.46*(144-N)^2 = N^2 Diff = 265.46*(144^2-288*N+N^2) - N^2 Diff = 0.208006944 N = 135.6729039 Xeg = 0.942172944 Stream 6 Toluene 144.00 Benzene 7.60 CH4 317.30 H2 735.40 Total = 1204.30 Stream 7 Streams 6 & 7 0.04 144.04 0.37 7.97 16.95 334.25 25.20 760.60 42.56 1246.86 Stoichiometric H2: quadratic equation in N 156 diff = 265*(144-N)(735.4-N) - (7.6+N)(317.3+N) abs(Diff) = 0.658055694 N= 143.5566045 Xeq = 0.996920866 143 No H2 or CH4 recycle: Accompanying CH4 = = 7.58 Diff = 265.46*(144^2-288*N+N^2) - (N+7.6)*(N+7.6) Diff 0.040094567 N = 135.2334102 Xeq = 0.939120904 Example 6.3 Equilibrium Conversion Streams 6 & 7 144.04 7.97 K(T) * (T)[H2) = [B] [CH4] Stream 6 Actual Feed T 600 C 265.46 Calc Kp 265.45 N abs(diff) 143.556593 0.0129681 Xeg 0.9969 yi Stream 6 Stream 7 0.1195715 Toluene 144.00 0.04 0.0063107 Benzene 7.60 0.37 0.2634726|CH4 317.30 16.95 0.6106452 H2 735.40 25.20 Total = 1 1204.30 42.56 + H, B + CH. 334.25 760.60 1246.86 5.106944444 stoichometric ratios Stream 6 Actual Feed N T 400C LHS = Kp 2332.10 Equilibrium Relation: Calc Kp 2331.52 N abs(diff) 143.949309 0.5770258 Xeg 0.9996 [B][CH] K,(T) = [T][H] K(T) - [T][H2) = [B] [CH] Xeg 94.22% 135.6735 0.942177 18407.31 18407,3 Or, , Thus, as P1, [T] and [H2] 1. LHS =265.5*(144-NX144-N) RHS = 10+N)"0+N) Abs(dift) = SO [B] and (CH41 0.011669 Stream 6 & 7 Actual Feed T 600C LHS = Rp 265.46 Calc Kp 265.45 N abs(diff) 143.597784 0.0175746 Xeg 0.9969 N 143.5567 For toluene: 0 99.69% 0.996921 69665.12 Xeg LHS-265.5*(144-N)[735.4-N) -(-NN RHS 7.6.N)"317.3N) Abs(dift) For H2: [H2) = n2N = PP.2/RT =P*Yu2/RT 69661.55 3.575489 Streams 6 & 7 Actual Feed T LHS = Kp RHS N abs(diff) 143.989453 0.4120418 Xeq 0.9999 Units: kmol/m' bar/[(bar.m/kmol.K)*K] R = 0.08314 bar.m/kmol.K 400C 2332.10 2331.68 Example 6.3 Equilibrium Conversion at 600 C Kp = 265.46 Stoichiometric H2: 265.46*(144-N)^2 = N^2 Diff = 265.46*(144^2-288*N+N^2) - N^2 Diff = 0.208006944 N = 135.6729039 Xeg = 0.942172944 Stream 6 Toluene 144.00 Benzene 7.60 CH4 317.30 H2 735.40 Total = 1204.30 Stream 7 Streams 6 & 7 0.04 144.04 0.37 7.97 16.95 334.25 25.20 760.60 42.56 1246.86 Stoichiometric H2: quadratic equation in N 156 diff = 265*(144-N)(735.4-N) - (7.6+N)(317.3+N) abs(Diff) = 0.658055694 N= 143.5566045 Xeq = 0.996920866 143 No H2 or CH4 recycle: Accompanying CH4 = = 7.58 Diff = 265.46*(144^2-288*N+N^2) - (N+7.6)*(N+7.6) Diff 0.040094567 N = 135.2334102 Xeq = 0.939120904








