Answered step by step
Verified Expert Solution
Question
1 Approved Answer
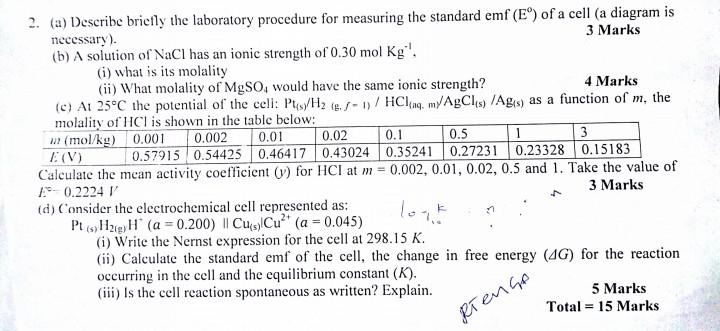
First and Second questions. please solve with proper explanation 0.01 2. (a) Describe briefly the laboratory procedure for measuring the standard emf (E) of a
First and Second questions. please solve with proper explanation
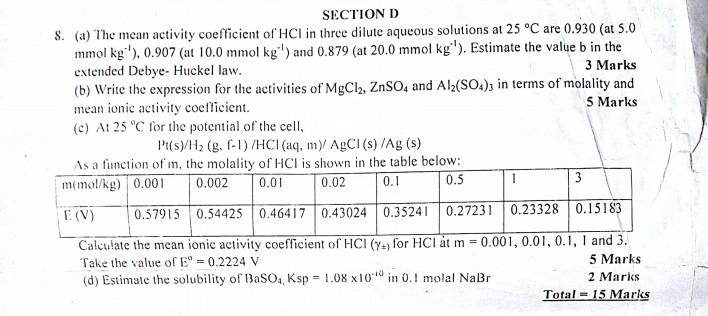
0.01 2. (a) Describe briefly the laboratory procedure for measuring the standard emf (E) of a cell (a diagram is necessary). 3 Marks (b) A solution of NaCl has an ionic strength of 0.30 mol kg'. (i) what is its molality (ii) What molality of MgSO, would have the same ionic strength? 4 Marks (c) At 25C the potential of the cell: Pts/H2 (8./ - 1) / HCl, myAgCl) /Ags as a function of m, the molality of HCl is shown in the table below: w (mol/kg) 0.001 0.002 0.02 0.1 0.5 1 3 (V) 0.57915 0.54425 0.46417 0.43024 0.35241 0.27231 0.23328 0.15183 Calculate the mean activity coefficient (1) for HCI at m = 0.002, 0.01, 0.02, 0.5 and 1. Take the value of - 0.2224 3 Marks (d) Consider the electrochemical cell represented as: P (Hag H (a=0.200) | CusCu?* (a = 0.045) (i) Write the Nernst expression for the cell at 298.15 K. (ii) Calculate the standard emf of the cell, the change in free energy (4G) for the reaction occurring in the cell and the equilibrium constant (K). (iii) is the cell reaction spontaneous as written? Explain. 5 Marks Total = 15 Marks () RTENGA SECTION D 8. (a) The mean activity coefficient of HCl in three dilute aqueous solutions at 25C are 0.930 (at 5.0 mmol kg ), 0.907 (at 10.0 mmol kg*') and 0.879 (at 20.0 mmol kg '). Estimate the value b in the extended Debye- Huckel law. 3 Marks (b) Write the expression for the activities of MgCl2, ZnSO and Alz(SO.), in terms of molality and mean ionic activity coefficient. 5 Marks (c) At 25 C for the potential of the cell, Pt(s)/H2(g. C-1) /HCl(aq, my AgCl (s) /Ag (5) As a function of in, the molality of HCI is shown in the table below: mmol/kg) 0.001 0.002 0.01 0.02 0.5 3 0.1 1 E(V) 0.57915 0.54425 0.46417 0.43024 0.35241 0.27231 0.23328 0.15183 Calculate the mean ionic activity coefficient of HCI (Y) for HCl t m -0.001, 0.01, 0.1, 1 and 3. Take the value of E = 0.2224 V 5 Marks (d) Estimate the solubility of BaSO4, Ksp = 1.08 x10 in 0.1 molal NaBr 2 Marks Total = 15 MarksStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


