Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Assume a nucleophile displaced a leaving group in both the alcohol and the protonated alcohol shown below. Using Nue for the nucleophile, draw
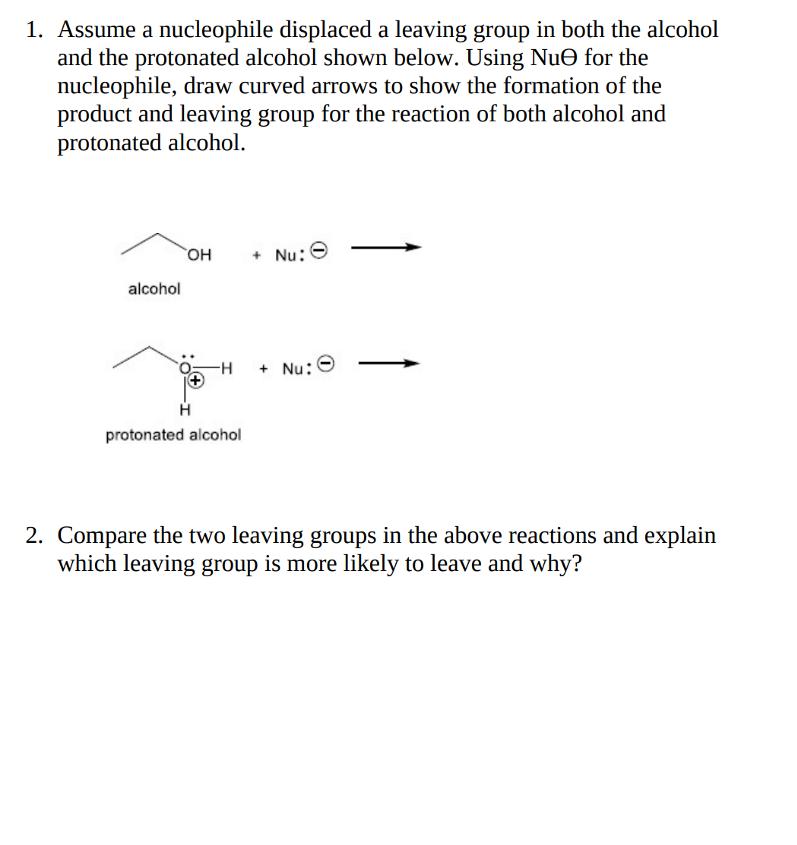
1. Assume a nucleophile displaced a leaving group in both the alcohol and the protonated alcohol shown below. Using Nue for the nucleophile, draw curved arrows to show the formation of the product and leaving group for the reaction of both alcohol and protonated alcohol. alcohol OH + Nu: -H + Nu: protonated alcohol 2. Compare the two leaving groups in the above reactions and explain which leaving group is more likely to leave and why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Sure I can help you solve this The leaving group in the reaction of the protonated alcohol is more likely to leave than the leaving group in the reaction of the alcohol This is because the oxygen atom ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


