Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For a chemical reaction that is spontaneous in the reverse direction, it is found that ASsys 0. This ear (Please note that in the
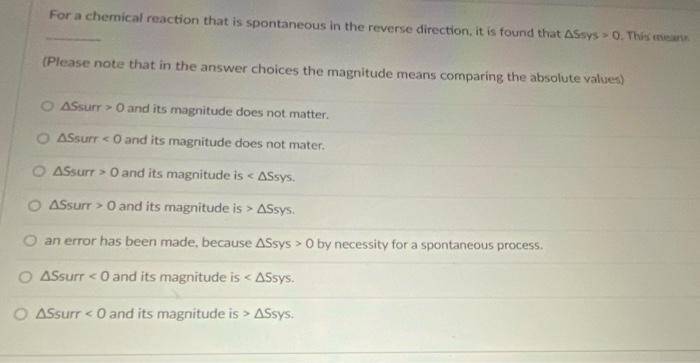
For a chemical reaction that is spontaneous in the reverse direction, it is found that ASsys 0. This ear (Please note that in the answer choices the magnitude means comparing the absolute values) 2 ASsurr > O and its magnitude does not matter. ASsurr < O and its magnitude does not mater. O ASsurr > 0 and its magnitude is < ASsys. O ASsurr > O and its magnitude is > ASsys. O an error has been made, because ASsys > 0 by necessity for a spontaneous process. O ASsurr ASsys.
Step by Step Solution
★★★★★
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
If S system 0 Then S surrounding 0 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63620442f3cc5_236384.pdf
180 KBs PDF File
63620442f3cc5_236384.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


