Question
For a K+Cl ion pair, attractive and repulsive energies EA and ER, respectively, depend on the distance between the ions r, according to EA =
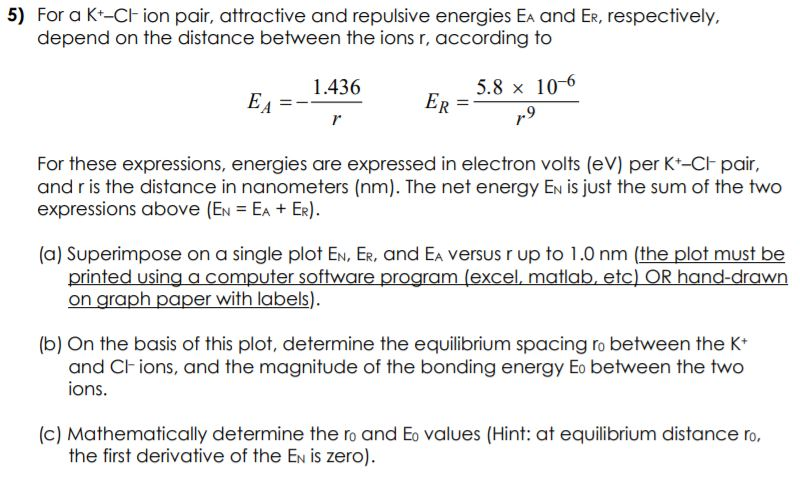
For a K+Cl ion pair, attractive and repulsive energies EA and ER, respectively, depend on the distance between the ions r, according to EA = 1.436 r ER = 5.8 106 r 9 For these expressions, energies are expressed in electron volts (eV) per K+Cl pair, and r is the distance in nanometers (nm). The net energy EN is just the sum of the two expressions above (EN = EA + ER). (a) Superimpose on a single plot EN, ER, and EA versus r up to 1.0 nm (the plot must be printed using a computer software program (excel, matlab, etc) OR hand-drawn on graph paper with labels). (b) On the basis of this plot, determine the equilibrium spacing r0 between the K+ and Cl ions, and the magnitude of the bonding energy E0 between the two ions. (c) Mathematically determine the r0 and E0 values (Hint: at equilibrium distance r0, the first derivative of the EN is zero).
5) For a Kt-Ch ion pair, attractive and repulsive energies EA and ER, respectively, depend on the distance between the ions r, according to 1.436 5.8 x 106 For these expressions, energies are expressed in electron volts (eV) per K+-Cl-pair, and r is the distance in nanometers (nm). The net energy Es is just the sum of the two expressions above (Ev = EA + ER). (a) Superimpose on a single plot EN, ER, and EA versus r up to 1.0 nm (the plot must be printed using a computer software program (excel, matlab.etc) OR hand-drawn on graph paper with labels). (b) On the basis of this plot, determine the equilibrium spacing ro between the K* and Ch ions, and the magnitude of the bonding energy Eo between the two ons (c) Mathematically determine the ro and Eo values (Hint: at equilibrium distance ro. the first derivative of the EN is zero)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


