Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For a reaction carried out as A B (k1), the reaction rate constant data against temperature are obtained and given below. Calculate the activation energy
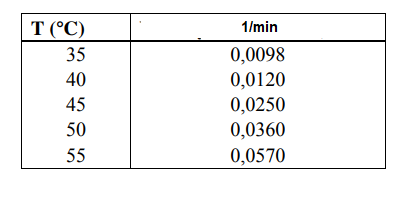
For a reaction carried out as A B (k1), the reaction rate constant data against temperature are obtained and given below. Calculate the activation energy and frequency factor required for this reaction to occur, using the graphical method.
\begin{tabular}{|r|c|} \hline T(C) & 1/min \\ \hline 35 & 0,0098 \\ 40 & 0,0120 \\ 45 & 0,0250 \\ 50 & 0,0360 \\ 55 & 0,0570 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


