Question
For below given chemical reactions: (1) 2Nis) + 02(9) = 2Ni0s) AG; = 471200 + 172T (J/mol) %3D (2) C(s) + 02(9) = CO2(9)
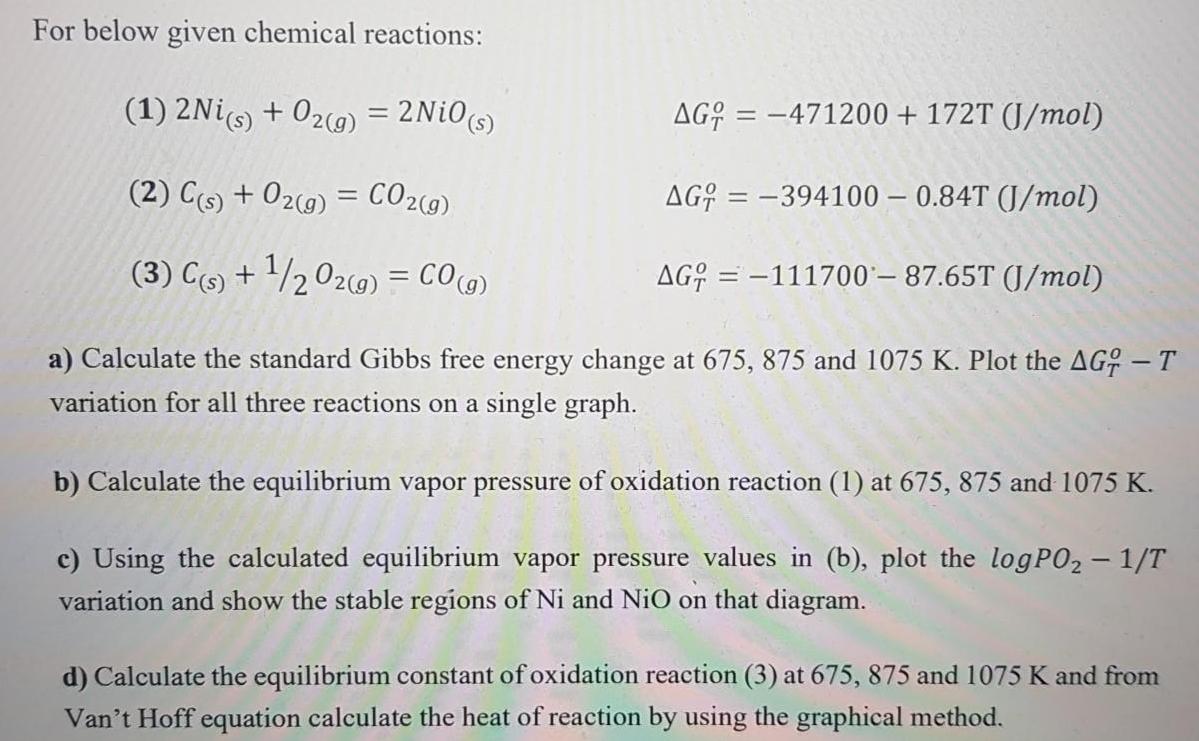
For below given chemical reactions: (1) 2Nis) + 02(9) = 2Ni0s) AG; = 471200 + 172T (J/mol) %3D (2) C(s) + 02(9) = CO2(9) AG; = -394100 0.84T (J/mol) %3D (3) C(9) + '/2 O2c9) = CO(g) AG; = -111700' 87.65T (J/mol) %3D a) Calculate the standard Gibbs free energy change at 675, 875 and 1075 K. Plot the AG; - T variation for all three reactions on a single graph. b) Calculate the equilibrium vapor pressure of oxidation reaction (1) at 675, 875 and 1075 K. c) Using the calculated equilibrium vapor pressure values in (b), plot the logPO2 1/T variation and show the stable regions of Ni and NiO on that diagram. d) Calculate the equilibrium constant of oxidation reaction (3) at 675, 875 and 1075 K and from Van't Hoff equation calculate the heat of reaction by using the graphical method.
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



