Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For each assigned molecule or polyatomic ion: (For the part with the table) Calculate the total number of valence electrons in the molecule. 2. Write
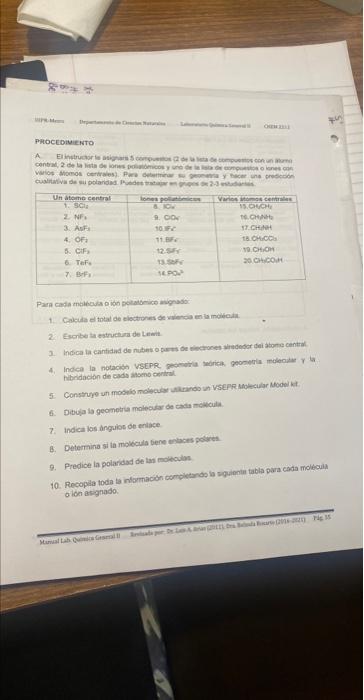

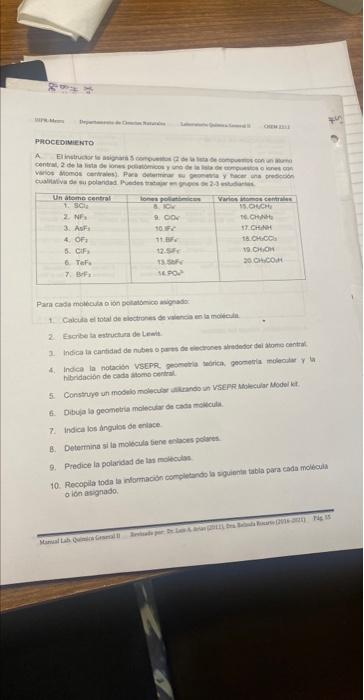
For each assigned molecule or polyatomic ion: (For the part with the table)
Calculate the total number of valence electrons in the molecule.
2. Write the Lewis structure.
3. Indicate the number of electron clouds or electron pairs around the central atom.
Indicate the VSEPR notation, theoretical geometry, molecular geometry and the hybridization of each atom.
hybridization of each central atom.
5.
Construct a molecular model using a VSEPR Molecular Model kit.
6.
Draw the molecular geometry of each molecule.
7. Indicate the bond angles.
8.
Determine if the molecule has polar bonds.
9. Predicts the polarity of the molecules.
10. Compile all the information by completing the following table for each molecule
or ion assigned
Quedtion 3
3
Consider the following
alcohols:
methanol (CH3OH);
ethanol (CHaCH2OH);
propane (CH3CH2CH2CH2OH); butanol (CHaCH2CH2CH2CH2OH).
Predict which of these
alcohols will be the best solvent for each of the following substances:
a.
b
C
Br2
KCI
CH3CH2CH2CH2CH2CH2CH3
CCIA

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


