Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For each bond drawn below, indicate the direction of bond polarity. Click in the blue box to toggle the arrow's direction; leave the box
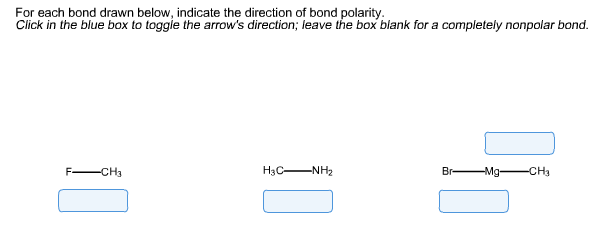
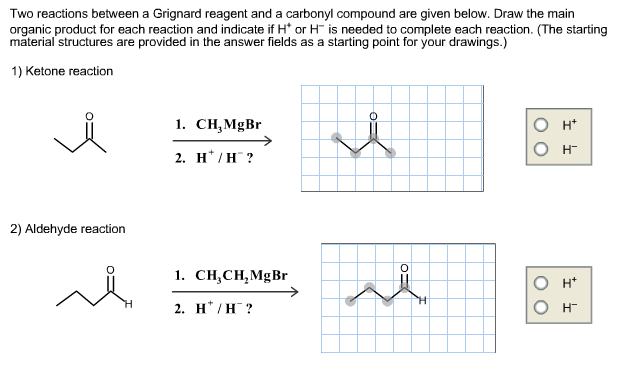
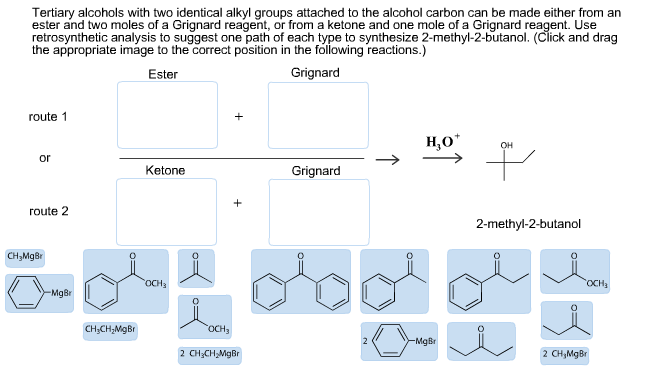
For each bond drawn below, indicate the direction of bond polarity. Click in the blue box to toggle the arrow's direction; leave the box blank for a completely nonpolar bond. F -CH3 H3C- -NH Br- Mg- -CH3 Two reactions between a Grignard reagent and a carbonyl compound are given below. Draw the main organic product for each reaction and indicate if H* or H is needed to complete each reaction. (The starting material structures are provided in the answer fields as a starting point for your drawings.) 1) Ketone reaction i 2) Aldehyde reaction ~ H 1. CH,MgBr 2. H*/H? 1. CHCHMgBr 2. H*/H? i 00 I I O H 00 II O H Tertiary alcohols with two identical alkyl groups attached to the alcohol carbon can be made either from an ester and two moles of a Grignard reagent, or from a ketone and one mole of a Grignard reagent. Use retrosynthetic analysis to suggest one path of each type to synthesize 2-methyl-2-butanol. (Click and drag the appropriate image to the correct position in the following reactions.) Ester Grignard route 1 or route 2 Ketone CHMgBr OCH -MgBr --- CHCHMgBr OCH3 2 CHCHMgBr Grignard 2 HO* -MgBr OH 2-methyl-2-butanol OCH 2 CHMgBr
Step by Step Solution
★★★★★
3.48 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
FCH3 HCNH ester ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


