Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For Each Compound Below, First Determine If It Is A Type I, Type II, Type III Or An Acid. Second, Name The Compound In The
For Each Compound Below, First Determine If It Is A Type I, Type II, Type III Or An Acid. Second, Name The Compound In The Second Blank. I) H2S Ii) Fe(CIO)3 Iii) FrF Iv) Sil4 Question 23 4 Pts Give Either The Name (If The Formula Is Provided) Or Formula (If The Name Is Provided) For The Following: I S?H6 Ii) Cadmium Bicarbonate Iii) Potassium Phosphide Iv)
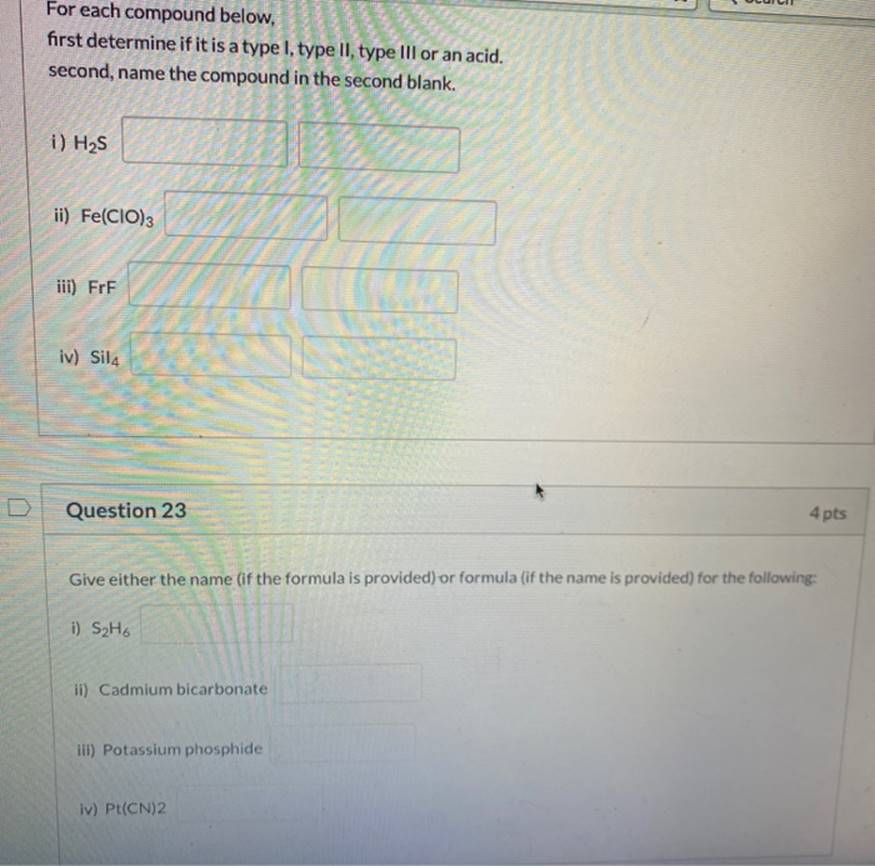
For each compound below, first determine if it is a type I, type II, type III or an acid. second, name the compound in the second blank. i) HS ii) Fe(CIO)3 iii) FrF iv) Sil 4 pts Question 23 Give either the name (if the formula is provided) or formula (if the name is provided) for the following: i) SH6 ii) Cadmium bicarbonate iii) Potassium phosphide iv) Pt(CN)2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


