Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For each of the Bronsted-Lowery acid base reactions below, draw the conjugate acid and conjugate base pairs most likely to form. (b) Circle the
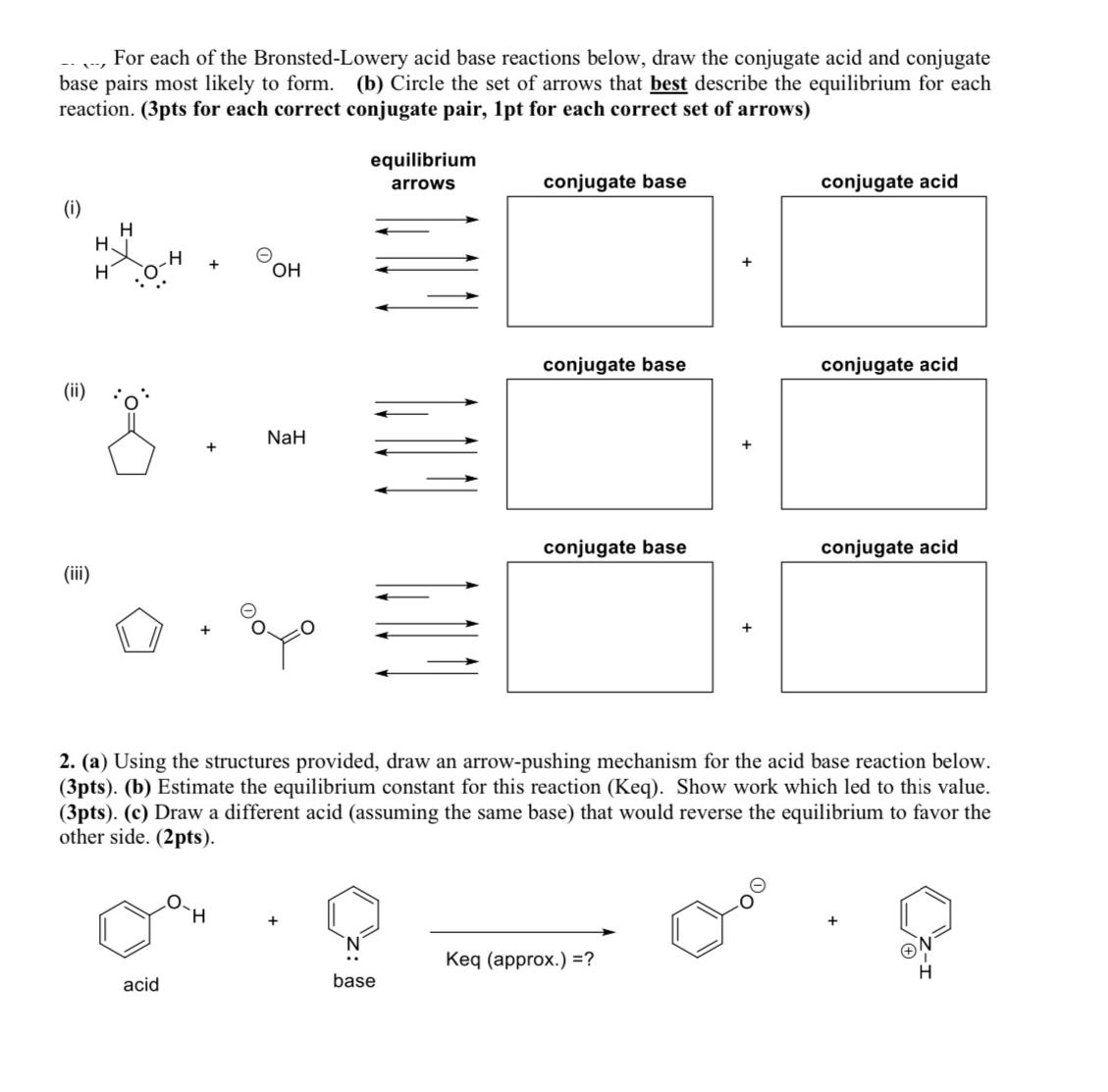
For each of the Bronsted-Lowery acid base reactions below, draw the conjugate acid and conjugate base pairs most likely to form. (b) Circle the set of arrows that best describe the equilibrium for each reaction. (3pts for each correct conjugate pair, 1pt for each correct set of arrows) (i) (iii) H H H OH NaH equilibrium arrows conjugate base conjugate acid conjugate base conjugate acid conjugate base conjugate acid 2. (a) Using the structures provided, draw an arrow-pushing mechanism for the acid base reaction below. (3pts). (b) Estimate the equilibrium constant for this reaction (Keq). Show work which led to this value. (3pts). (c) Draw a different acid (assuming the same base) that would reverse the equilibrium to favor the other side. (2pts). acid H + N' base Keq (approx.) =?
Step by Step Solution
★★★★★
3.41 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
i H2O H3O H3O OH H3O conjugate acid OH conjugate base ii H Na NaH H H conjugate acid ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


