Answered step by step
Verified Expert Solution
Question
1 Approved Answer
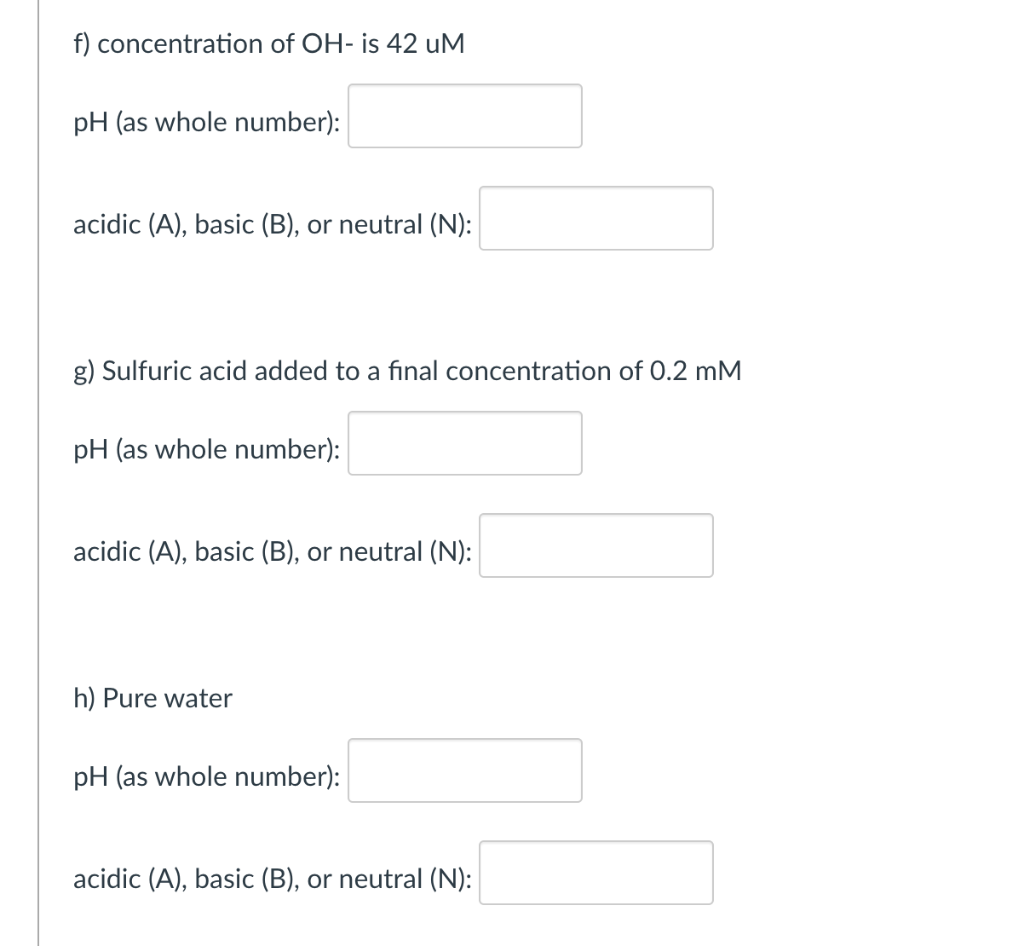
For each of the following solutions, calculate its pH and say whether the solution is acidic, basic, or neutral. When you enter your pH answer,

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


