Question
Suppose you performed a similar experiment using diphenyl ether as your solvent. Given the data in the table, what would be the freezing point
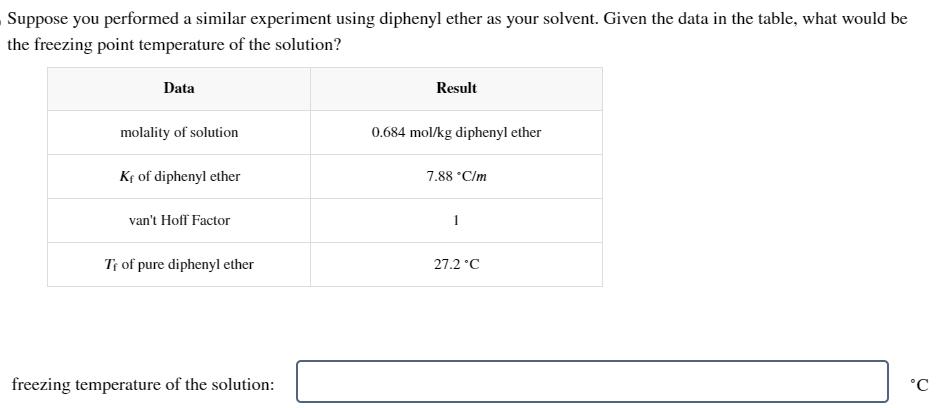
Suppose you performed a similar experiment using diphenyl ether as your solvent. Given the data in the table, what would be the freezing point temperature of the solution? Data Result molality of solution 0.684 mol/kg diphenyl ether Kf of diphenyl ether van't Hoff Factor T of pure diphenyl ether freezing temperature of the solution: 7.88 C/m 27.2C C
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
To determine the freezing point temperature of the solution we can use the equation T Kf m i Wh...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Fluid Mechanics
Authors: Robert L. Mott, Joseph A. Untener
7th edition
132558920, 9780133414622 , 978-0132558921
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



