Question
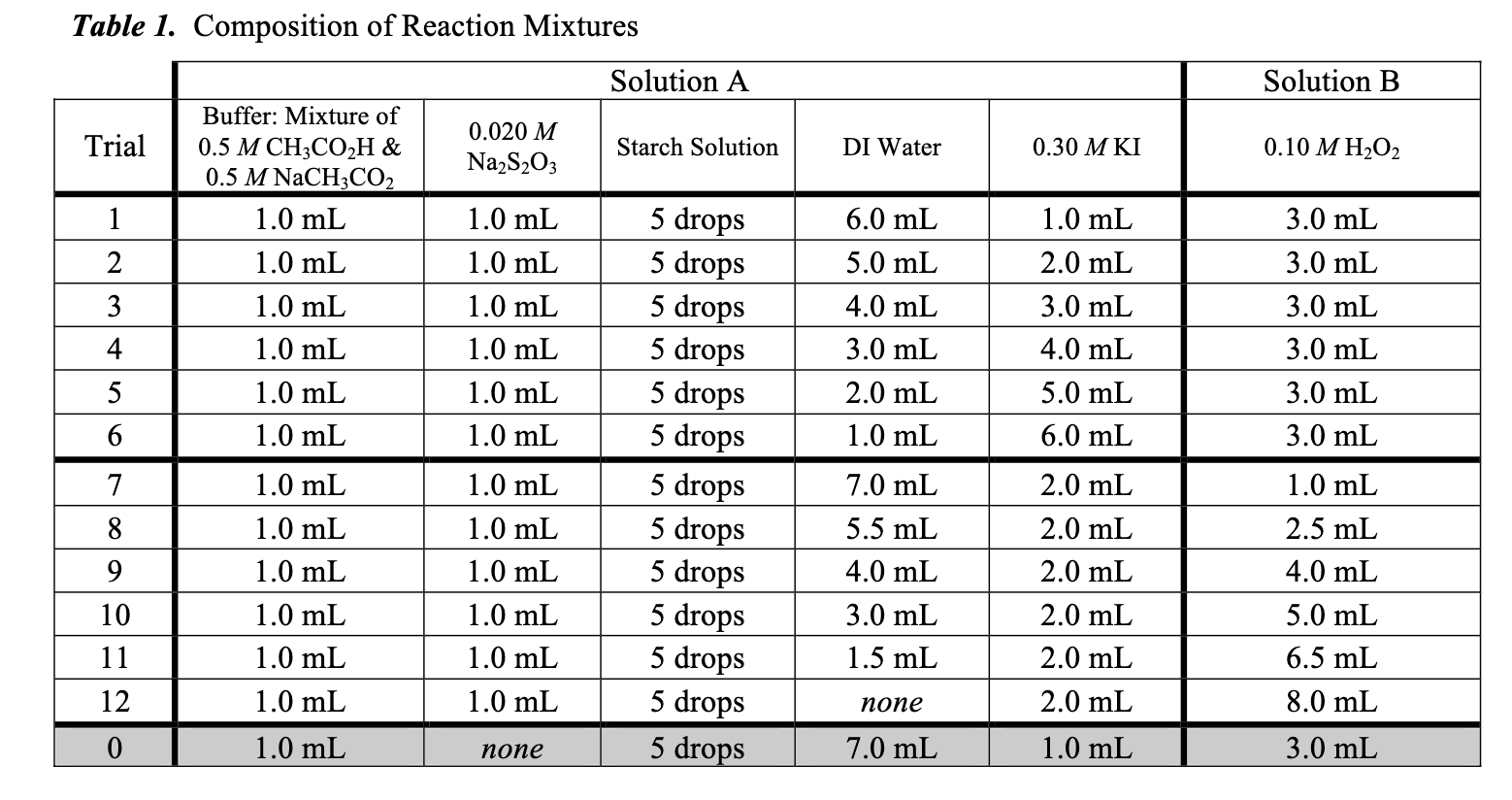
For each trial, calculate the initial concentrations of hydrogen peroxide [H2O2]0 and iodide [I-]0 as well as log[H2O2]0 and log[I-]0. Select two trials in which
For each trial, calculate the initial concentrations of hydrogen peroxide [H2O2]0 and iodide [I-]0 as well as log[H2O2]0 and log[I-]0.
Select two trials in which the [I-]0 is constant between trials. Analyze the difference in the [H2O2]0 and the rate of reaction to make a preliminary determination of the order of the reaction for [H2O2].
Select two trials in which the [H2O2]0 is constant between trials. Analyze the difference in the [I-]0 and the rate of reaction to make a preliminary determination of the order of the reaction for [I-].
Chemical net equation: 2 I- (aq) + H2O2 (aq) + 2H3O+ (aq) I2 (aq) + 4H2O (l)
Rate: 4.00 10^-6 M/s
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


