For jet aircraft flying out of Houston on a summer afternoon (T = 95 F, 70% relative humidity) and Palm Springs on a winter

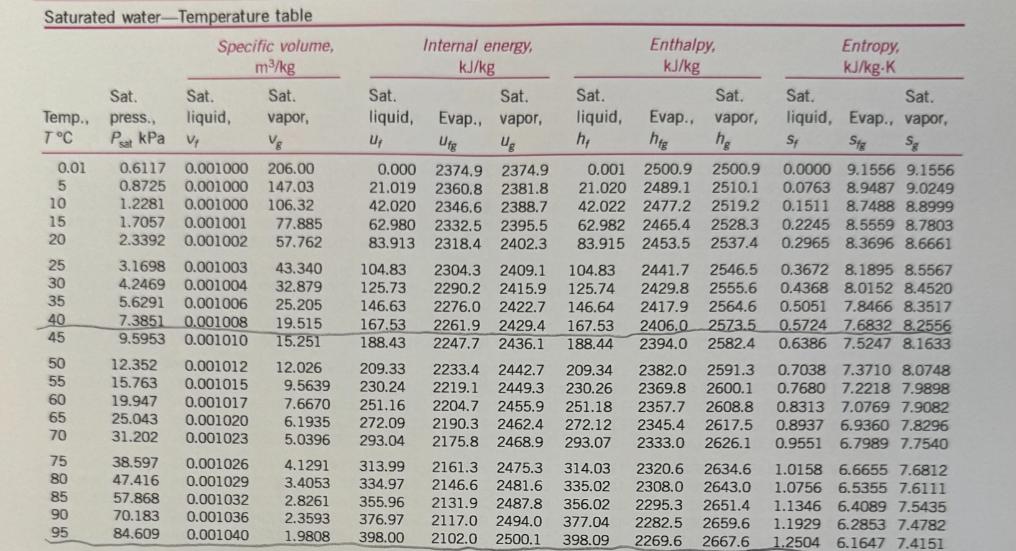
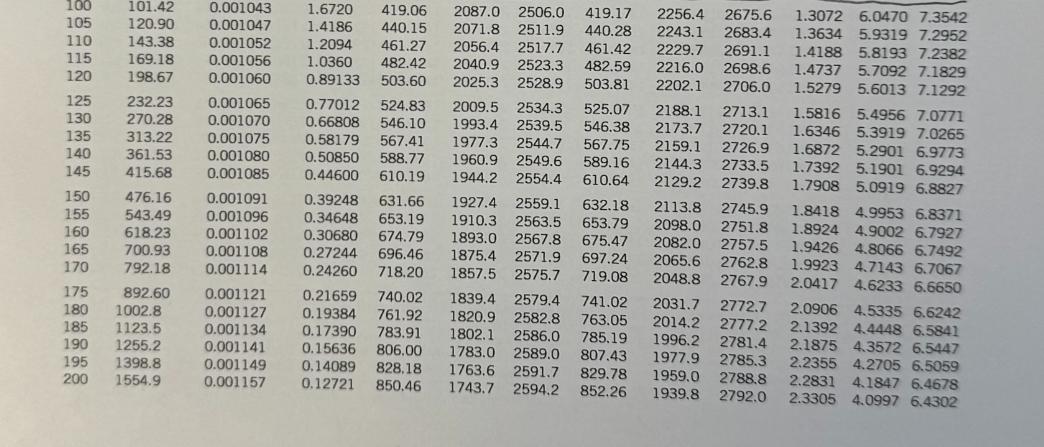
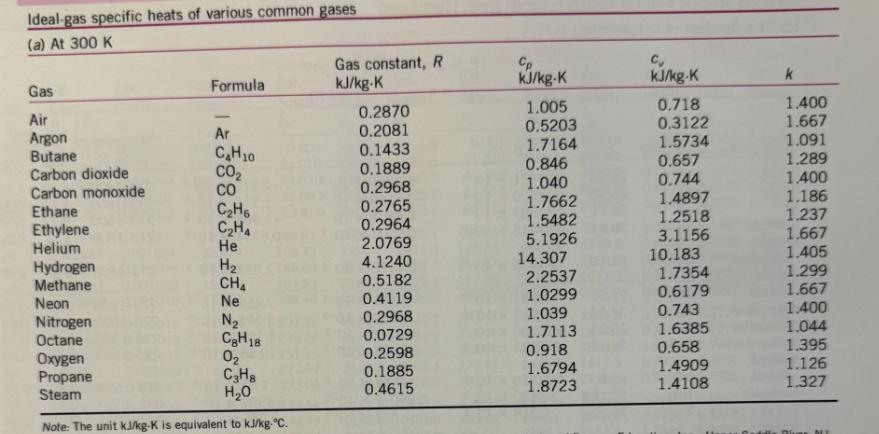
For jet aircraft flying out of Houston on a summer afternoon (T = 95 F, 70% relative humidity) and Palm Springs on a winter morning (T = 40 F, 55% relative humidity), determine for both locations: a) the mass of water vapor in 1 m of air b) the thermal efficiency of an ideal Brayton cycle with a pressure ratio of 9. Assume that both cities are at sea level and both air and HO can be treated as ideal gases with constant specific heats evaluated at 300 K. Saturated water-Temperature table Specific volume, m/kg Sat. Sat. Sat. Temp., press., liquid, vapor, TC Psat kPa V Vg 0.01 BARR BARN4 888 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 0.6117 0.001000 206.00 0.8725 0.001000 147.03 1.2281 0.001000 106.32 1.7057 0.001001 77.885 2.3392 0.001002 57.762 3.1698 0.001003 43.340 4.2469 0.001004 32.879 5.6291 0.001006 25.205 7.3851 0.001008 19.515 9.5953 0.001010 15.251 12.352 15.763 19.947 25.043 31.202 0.001012 12.026 0.001015 0.001017 0.001020 0.001023 38.597 0.001026 47.416 0.001029 57.868 0.001032 70.183 0.001036 84.609 0.001040 Internal energy, kJ/kg Sat. liquid, Evap., Ufg U 209.33 9.5639 230.24 7.6670 251.16 272.09 293.04 6.1935 5.0396 4.1291 313.99 3.4053 334.97 2.8261 355.96 2.3593 376.97 1.9808 398.00 Sat. vapor, Ug Sat. liquid, h Enthalpy, kJ/kg Sat. Evap., vapor, hfg hg Entropy, kJ/kg-K 0.001 2500.9 2500.9 21.020 2489.1 2510.1 42.022 2477.2 2519.2 62.982 2465.4 2528.3 83.915 2453.5 2537.4 0.000 2374.9 2374.9 21.019 2360.8 2381.8 42.020 2346.6 2388.7 62.980 2332.5 2395.5 83.913 2318.4 2402.3 104.83 2304.3 2409.1 104.83 2441.7 2546.5 125.73 2290.2 2415.9 125.74 2429.8 2555.6 146.63 2276.0 2422.7 146.64 2417.9 2564.6 167.53 2261.9 2429.4 167.53 2406.0 2573.5 188.43 2247.7 2436.1 188.44 2394.0 2582.4 2233.4 2442.7 209.34 2382.0 2591.3 0.7038 7.3710 8.0748 2219.1 2449.3 230.26 2369.8 2600.1 0.7680 7.2218 7.9898 2204.7 2455.9 251.18 2357.7 2608.8 0.8313 7.0769 7.9082 2190.3 2462.4 272.12 2345.4 2617.5 0.8937 6.9360 7.8296 2175.8 2468.9 293.07 2333.0 2626.1 0.9551 6.7989 7.7540 2161.3 2475.3 314.03 2320.6 2634.6 1.0158 6.6655 7.6812 2146.6 2481.6 335.02 2308.0 2643.0 1.0756 6.5355 7.6111 2131.9 2487.8 356.02 2295.3 2651.4 2117.0 2494.0 377.04 2282.5 2659.6 2102.0 2500.1 398.09 2269.6 2667.6 1.1346 6.4089 7.5435 1.1929 6.2853 7.4782 1.2504 6.1647 7.4151 Sat. Sat. liquid, Evap., vapor, St Sig Sg 0.0000 9.1556 9.1556 0.0763 8.9487 9.0249 0.1511 8.7488 8.8999 0.2245 8.5559 8.7803 0.2965 8.3696 8.6661 0.3672 8.1895 8.5567 0.4368 8.0152 8.4520 0.5051 7.8466 8.3517 0.5724 7.6832 8.2556 0.6386 7.5247 8.1633 100 105 110 115 120 125 130 135 140 145 101.42 120.90 0.001043 0.001047 0.001052 169.18 0.001056 143.38 198.67 0.001060 232.23 270.28 0.001065 0.001070 0.001075 0.001080 415.68 0.001085 313.22 361.53 160 150 476.16 0.001091 155 543.49 0.001096 618.23 0.001102 165 700.93 0.001108 170 792.18 0.001114 175 892.60 0.001121 180 1002.8 0.001127 185 1123.5 190 1255.2 0.001134 0.001141 0.001149 0.001157 195 1398.8 200 1554.9 1.6720 419.06 2087.0 2506.0 419.17 2256.4 2675.6 1.4186 440.15 2071.8 2511.9 440.28 2243.1 2683.4 1.2094 461.27 2056.4 2517.7 461.42 2229.7 2691.1 1.0360 482.42 2040.9 2523.3 482.59 2216.0 2698.6 0.89133 503.60 2025.3 2528.9 503.81 2202.1 2706.0 2009.5 2534.3 525.07 2188.1 2713.1 1993.4 2539.5 546.38 2173.7 2720.1 1977.3 2544.7 567.75 2159.1 2726.9 1960.9 2549.6 589.16 2144.3 2733.5 1944.2 2554.4 610.64 2129.2 2739.8 1927.4 2559.1 632.18 2113.8 2745.9 1910.3 2563.5 653.79 2098.0 2751.8 1893.0 2567.8 675.47 2082.0 2757.5 1875.4 2571.9 697.24 2065.6 2762.8 1857.5 2575.7 719.08 2048.8 2767.9 1839.4 2579.4 741.02 2031.7 2772.7 1820.9 2582.8 763.05 2014.2 2777.2 1802.1 2586.0 785.19 1996.2 2781.4 1783.0 2589.0 807.43 1977.9 2785.3 1763.6 2591.7 829.78 1959.0 2788.8 1743.7 2594.2 852.26 1939.8 2792.0 0.77012 524.83 0.66808 546.10 0.58179 567.41 0.50850 588.77 0.44600 610.19 0.39248 631.66 0.34648 653.19 0.30680 674.79 0.27244 696.46 0.24260 718.20 0.21659 740.02 0.19384 761.92 0.17390 783.91 0.15636 806.00 0.14089 828.18 0.12721 850.46 1.3072 6.0470 7.3542 1.3634 5.9319 7.2952 1.4188 5.8193 7.2382 1.4737 5.7092 7.1829 1.5279 5.6013 7.1292 1.5816 5.4956 7.0771 1.6346 5.3919 7.0265 1.6872 5.2901 6.9773 1.7392 5.1901 6.9294 1.7908 5.0919 6.8827 1.8418 4.9953 6.8371 1.8924 4.9002 6.7927 1.9426 4.8066 6.7492 1.9923 4.7143 6.7067 2.0417 4.6233 6.6650 2.0906 4.5335 6.6242 2.1392 4.4448 6.5841 2.1875 4.3572 6.5447 2.2355 4.2705 6.5059 2.2831 4.1847 6.4678 2.3305 4.0997 6.4302 Ideal-gas specific heats of various common gases (a) At 300 K Gas Air Argon Butane Carbon dioxide Carbon monoxide Ethane Ethylene Helium Hydrogen Methane Neon Nitrogen Octane Formula Oxygen Propane Steam Ar CH10 CO CO CH6 CH4 He H CH Ne N CH18 0 C3H8 HO Note: The unit kJ/kg-K is equivalent to kj/kg-C. Gas constant, R kJ/kg-K 0.2870 0.2081 0.1433 0.1889 0.2968 0.2765 0.2964 2.0769 4.1240 0.5182 0.4119 0.2968 0.0729 0.2598 0.1885 0.4615 Cp kJ/kg-K 1.005 0.5203 1.7164 0.846 1.040 1.7662 1.5482 5.1926 14.307 2.2537 1.0299 1.039 1.7113 0.918 1.6794 1.8723 Cu kJ/kg-K 0.718 0.3122 1.5734 0.657 0.744 1.4897 1.2518 3.1156 10.183 1.7354 0.6179 0.743 1.6385 0.658 1.4909 1.4108 k 1.400 1.667 1.091 1.289 1.400 1.186 1.237 1.667 1.405 1.299 1.667 1.400 1.044 1.395 1.126 1.327 NE
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Part a Mass of Water Vapor in 1 m of Air Houston Temperature conversion T1 95F 35C 308 K Vapor press...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


