Answered step by step
Verified Expert Solution
Question
1 Approved Answer
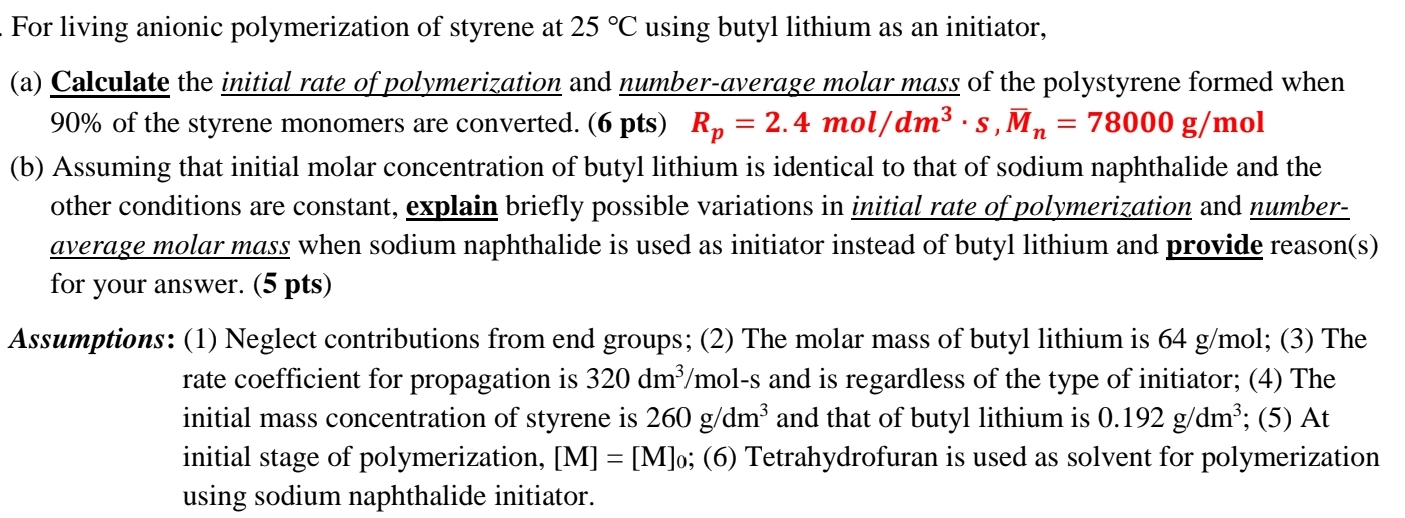
For living anionic polymerization of styrene at 2 5 C using butyl lithium as an initiator, ( a ) Calculate the initial rate of polymerization
For living anionic polymerization of styrene at using butyl lithium as an initiator,
a Calculate the initial rate of polymerization and numberaverage molar mass of the polystyrene formed when of the styrene monomers are converted. pts
b Assuming that initial molar concentration of butyl lithium is identical to that of sodium naphthalide and the other conditions are constant, explain briefly possible variations in initial rate of polymerization and numberaverage molar mass when sodium naphthalide is used as initiator instead of butyl lithium and provide reasons for your answer. pts
Assumptions: Neglect contributions from end groups; The molar mass of butyl lithium is ; The rate coefficient for propagation is and is regardless of the type of initiator; The initial mass concentration of styrene is and that of butyl lithium is ; At initial stage of polymerization, ; Tetrahydrofuran is used as solvent for polymerization using sodium naphthalide initiator.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


