Question
For N=20, compute: A gas mixture consists of nitrogen, with a volume in the normal state conditions of VN, N2 = (0.79 +0.001N) mN
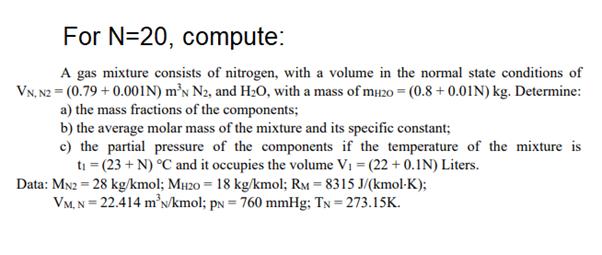
For N=20, compute: A gas mixture consists of nitrogen, with a volume in the normal state conditions of VN, N2 = (0.79 +0.001N) mN N2, and H0, with a mass of mH20 = (0.8 +0.01N) kg. Determine: a) the mass fractions of the components; b) the average molar mass of the mixture and its specific constant; c) the partial pressure of the components if the temperature of the mixture is t = (23+ N) C and it occupies the volume V =(22+0.1N) Liters. Data: MN2 = 28 kg/kmol; MH20 = 18 kg/kmol; RM = 8315 J/(kmol-K); VM, N = 22.414 mN/kmol; pN = 760 mmHg; TN = 273.15K.
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



