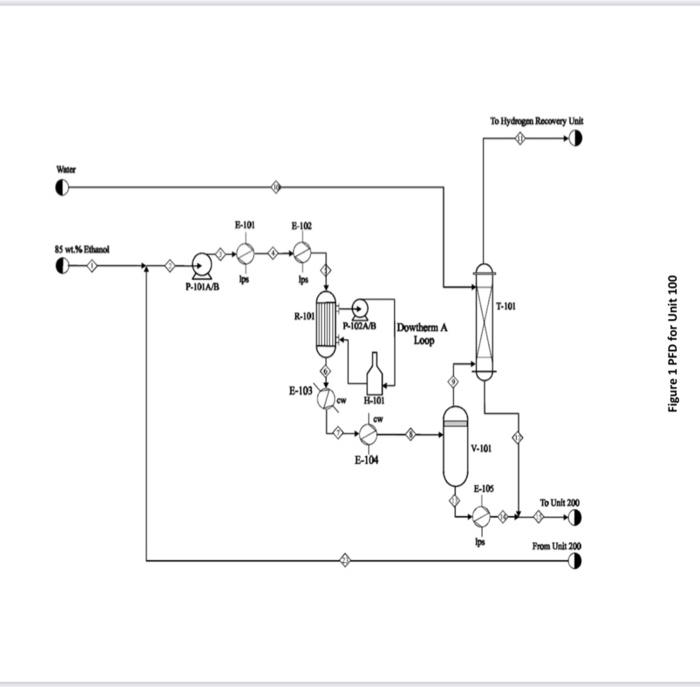
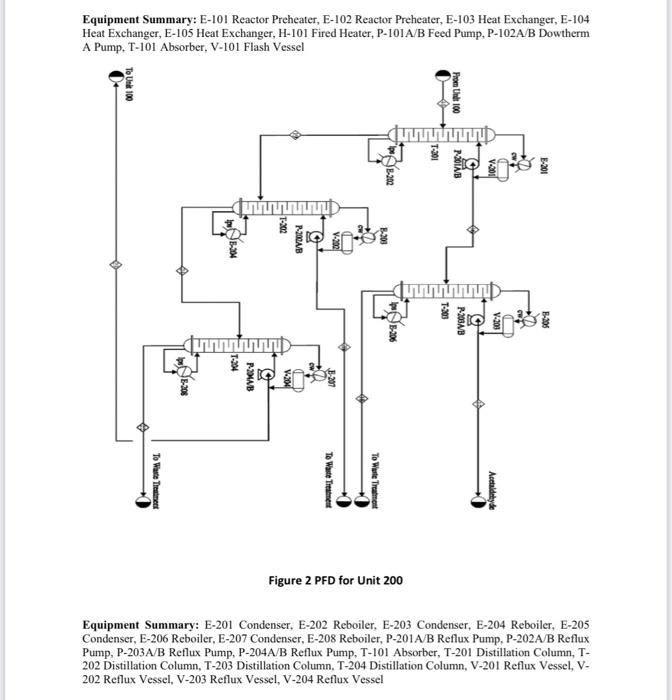
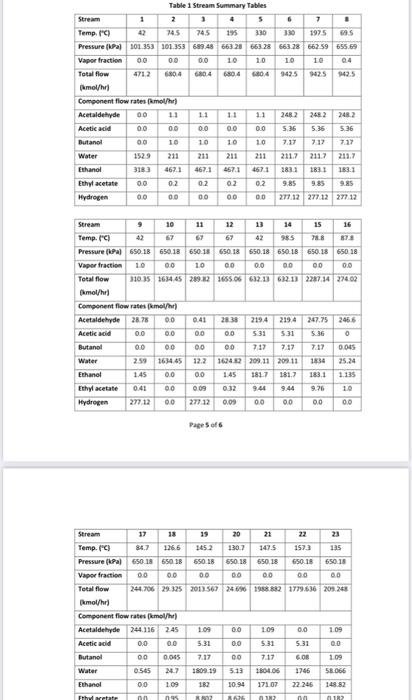
for plant design course .. i need the solution for part 2 and 8 only please

2CH3CH2OHCH3COOC4H1+2H4 Lthanol Ithyl Acrtate + Hydresen ZCHCH6OHCHACHAOH+HOIthanolButanel+WaterCH1CH1OH+H2OCH,COOH+2H2tthanal+WaterAcreticAcid+Hydraten Generally, the ethanol detydration into ethylene is at endothermic reaction (thermodymamically and kinetically controlled). The main side reaction of ethanol debydration into diectyy ether is a sighty exbthermic reaction. With the use of Figure 1 and 2 and stream summary Tablei. 1. Draw a schematic BFD for the process lyou need to display dinections and the main inlet. outlet and rectycle camponent. 2. Suggest two possible alternatives flowheet for heat interation network foased on the achematic BbD. 3. Determine (i) The single - pass comversion and overall conversion of ethanel. (i0) The seiectivity of ethanol for echyl acete. (iii) The yield for butand 4. Eutimate the mass flow rate of the lps for Eido2. 5. Futimace the Bro of P-101A/A. 6. Justify the use of V101 and T101 7. Juztify the operational conditions (top preisure at 650 . 18 wha and g4.706 for 7-203. B. Plot a simple control loop to control: a. The remperature of the feed going into y10. b. The pressure of stream No.3. 9. Eqaluate the prefic margin for the procesifuse the spithicmetry al the neaction as your basus. (iii) The yield for butanol 4. Estimate the mass flow rate of the lps for E102. 5. Estimate the BHP of P101A/B. 6. Justify the use of V101 and T101. 7. Justify the operational conditions (top pressure at 650.18kPa and 84.7C ) for T203. 8. Plot a simple control loop to control: a. The temperature of the feed going into 101. b. The pressure of stream No.3. 9. Evaluate the profit margin for the process (use the stoichiometry of the reaction as your basis). *Year 2008 (Table 8.3 text book) Equipment Summary: E-101 Reactor Preheater, E-102 Reactor Preheater, E-103 Heat Exchanger, E-104 Heat Exchanger. E-105 Heat Exchanger. H-101 Fired Heater. P-101A/B Feed Pumb. P-102A/B Dowtherm Figure 2 PFD for Unit 200 Equipment Summary: E-201 Condenser, E-202 Reboiler, E-203 Condenser, E-204 Reboiler, E-205 Condenser, E-206 Reboiler, E-207 Condenser, E-208 Reboiler, P-201A/B Reflux Pump, P-202A/B Reflux Pump, P-203A/B Reflux Pump, P-204A/B Reflux Pump, T-101 Absorber, T-201 Distillation Column, T202 Distillation Column, T-203 Distillation Column, T-204 Distillation Column, V-201 Reflux Vessel, V202 Reflux Vessel, V-203 Reflux Vessel, V-204 Reflux Vessel 2CH3CH2OHCH3COOC4H1+2H4 Lthanol Ithyl Acrtate + Hydresen ZCHCH6OHCHACHAOH+HOIthanolButanel+WaterCH1CH1OH+H2OCH,COOH+2H2tthanal+WaterAcreticAcid+Hydraten Generally, the ethanol detydration into ethylene is at endothermic reaction (thermodymamically and kinetically controlled). The main side reaction of ethanol debydration into diectyy ether is a sighty exbthermic reaction. With the use of Figure 1 and 2 and stream summary Tablei. 1. Draw a schematic BFD for the process lyou need to display dinections and the main inlet. outlet and rectycle camponent. 2. Suggest two possible alternatives flowheet for heat interation network foased on the achematic BbD. 3. Determine (i) The single - pass comversion and overall conversion of ethanel. (i0) The seiectivity of ethanol for echyl acete. (iii) The yield for butand 4. Eutimate the mass flow rate of the lps for Eido2. 5. Futimace the Bro of P-101A/A. 6. Justify the use of V101 and T101 7. Juztify the operational conditions (top preisure at 650 . 18 wha and g4.706 for 7-203. B. Plot a simple control loop to control: a. The remperature of the feed going into y10. b. The pressure of stream No.3. 9. Eqaluate the prefic margin for the procesifuse the spithicmetry al the neaction as your basus. (iii) The yield for butanol 4. Estimate the mass flow rate of the lps for E102. 5. Estimate the BHP of P101A/B. 6. Justify the use of V101 and T101. 7. Justify the operational conditions (top pressure at 650.18kPa and 84.7C ) for T203. 8. Plot a simple control loop to control: a. The temperature of the feed going into 101. b. The pressure of stream No.3. 9. Evaluate the profit margin for the process (use the stoichiometry of the reaction as your basis). *Year 2008 (Table 8.3 text book) Equipment Summary: E-101 Reactor Preheater, E-102 Reactor Preheater, E-103 Heat Exchanger, E-104 Heat Exchanger. E-105 Heat Exchanger. H-101 Fired Heater. P-101A/B Feed Pumb. P-102A/B Dowtherm Figure 2 PFD for Unit 200 Equipment Summary: E-201 Condenser, E-202 Reboiler, E-203 Condenser, E-204 Reboiler, E-205 Condenser, E-206 Reboiler, E-207 Condenser, E-208 Reboiler, P-201A/B Reflux Pump, P-202A/B Reflux Pump, P-203A/B Reflux Pump, P-204A/B Reflux Pump, T-101 Absorber, T-201 Distillation Column, T202 Distillation Column, T-203 Distillation Column, T-204 Distillation Column, V-201 Reflux Vessel, V202 Reflux Vessel, V-203 Reflux Vessel, V-204 Reflux Vessel