Question
For radium-226 (atomic mass = 226.0254 u) obtain (a) the mass defect, (b) the binding energy in MeV, and (c) the binding energy per
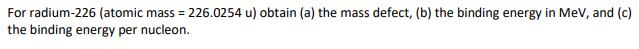
For radium-226 (atomic mass = 226.0254 u) obtain (a) the mass defect, (b) the binding energy in MeV, and (c) the binding energy per nucleon.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Mass Defect m Expected mass of Ra226 Expected mass 88 protons 1007276u 138 neutrons 1008665 u 88...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: David Young, Shane Stadler
10th edition
1118486897, 978-1118836873, 1118836871, 978-1118899205, 1118899202, 978-1118486894
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



