Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For solidification of Iron (Fe), Latent heat of fusion is Lv=1.85109J/m3; interfacial energy: =0.204J/m2; melting point is Tm=1811K; and T=295K; Lattice parameter of Fe:a=0.292nm -
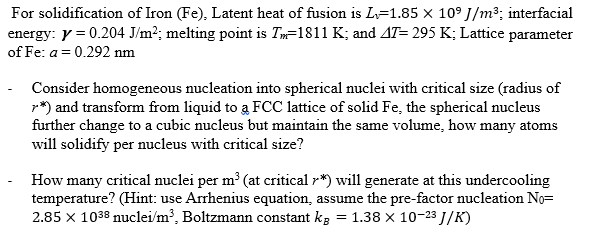 For solidification of Iron (Fe), Latent heat of fusion is Lv=1.85109J/m3; interfacial energy: =0.204J/m2; melting point is Tm=1811K; and T=295K; Lattice parameter of Fe:a=0.292nm - Consider homogeneous nucleation into spherical nuclei with critical size (radius of r) and transform from liquid to a FCC lattice of solid Fe, the spherical nucleus further change to a cubic nucleus but maintain the same volume, how many atoms will solidify per nucleus with critical size? - How many critical nuclei per m3 (at critical r ) will generate at this undercooling temperature? (Hint: use Arrhenius equation, assume the pre-factor nucleation N0= 2.851038 nucle /m3, Boltzmann constant kB=1.381023J/K )
For solidification of Iron (Fe), Latent heat of fusion is Lv=1.85109J/m3; interfacial energy: =0.204J/m2; melting point is Tm=1811K; and T=295K; Lattice parameter of Fe:a=0.292nm - Consider homogeneous nucleation into spherical nuclei with critical size (radius of r) and transform from liquid to a FCC lattice of solid Fe, the spherical nucleus further change to a cubic nucleus but maintain the same volume, how many atoms will solidify per nucleus with critical size? - How many critical nuclei per m3 (at critical r ) will generate at this undercooling temperature? (Hint: use Arrhenius equation, assume the pre-factor nucleation N0= 2.851038 nucle /m3, Boltzmann constant kB=1.381023J/K ) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


