Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For steady state flow through a throttling valve with negligible changes in kinetic and potential energies, the first law states that the enthalpy remains constant,
For steady state flow through a throttling valve with negligible changes in kinetic and potential energies, the first law states that the enthalpy remains constant, that is, the isenthalpic process. The variation of temperature with pressure in such a process is known as the Joule-Thomson coefficient, . Show that the Joule-Thomson coefficient is given by (check the image). 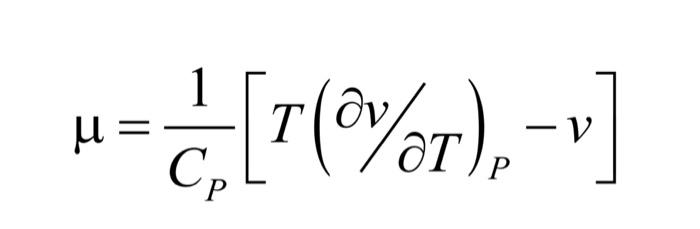
=CP1[T(v/T)Pv] The throttling process can cause an increase or decrease in temperature depending on the value of . Answer: What happens when 0?
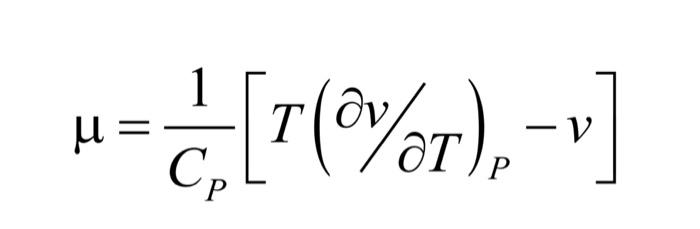
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


