Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the cell shown, the measured cell potential, Ecell, is -0.3605 V at 25 C. Pt(s) | H, (g, 0.881 atm) | H*(aq, ?
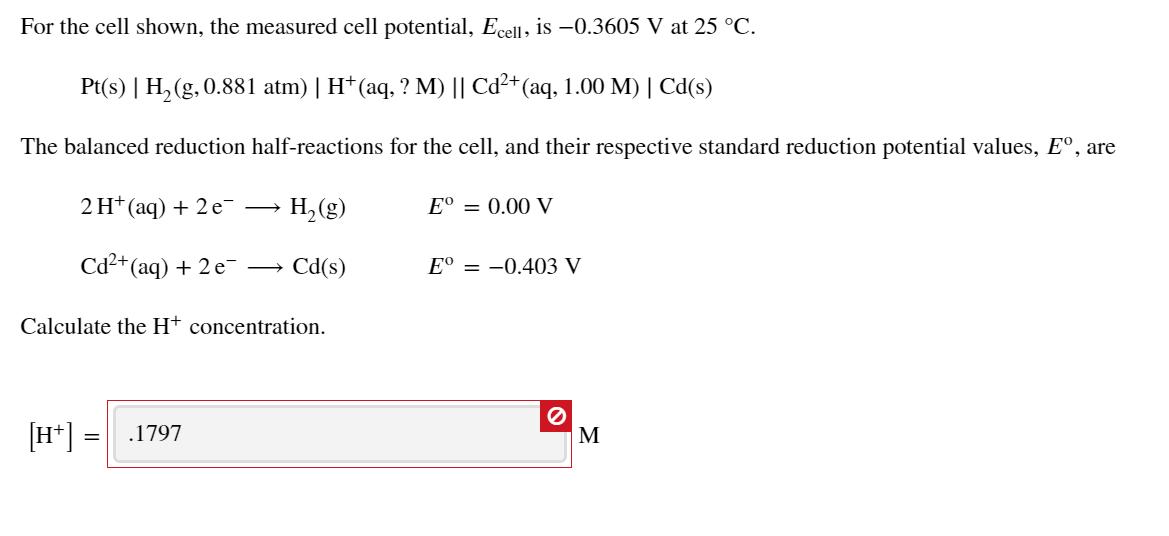
For the cell shown, the measured cell potential, Ecell, is -0.3605 V at 25 C. Pt(s) | H, (g, 0.881 atm) | H*(aq, ? M) || Cd+ (aq, 1.00 M) | Cd(s) The balanced reduction half-reactions for the cell, and their respective standard reduction potential values, E, are 2 H* (aq) + 2 e H, (g) E = 0.00 V Cd2+(aq) + 2 e Cd(s) E = -0.403 V Calculate the Ht concentration. [H*] = .1797 M
Step by Step Solution
★★★★★
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Reduction half cell deachions 24 ag 2e Ha g Cd2 ag 2e c...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


