Question: For the following systems, evaluate the boundary condition at the interface as mol% and wt%. a.) Air-water system at 75F with P = 760

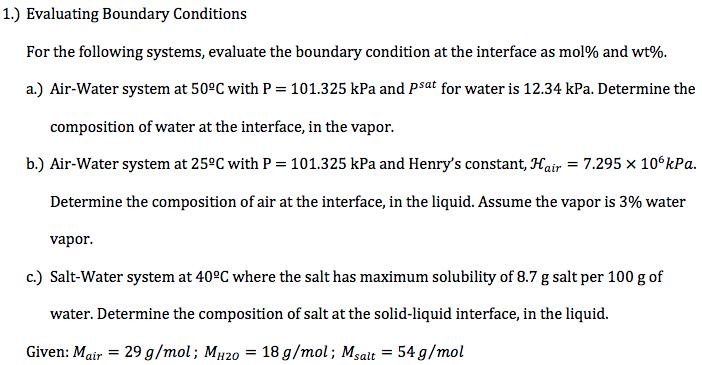
For the following systems, evaluate the boundary condition at the interface as mol% and wt%. a.) Air-water system at 75F with P = 760 mmHg and psat for water is 22.5 mmHg. Determine the composition of water at the interface, in the vapor. For this step, assume the liquid is pure water. b.) Air-water system at 75F with P 101.325 kPa. Determine the composition of O2 at the interface, 4.71 106kPa and the composition of O2 in humid air is 20 mol%. in the liquid. Let H02 = = 1.) Evaluating Boundary Conditions For the following systems, evaluate the boundary condition at the interface as mol% and wt%. a.) Air-Water system at 50C with P = 101.325 kPa and psat for water is 12.34 kPa. Determine the composition of water at the interface, in the vapor. b.) Air-Water system at 25C with P = 101.325 kPa and Henry's constant, Hair = 7.295 106kPa. Determine the composition of air at the interface, in the liquid. Assume the vapor is 3% water vapor. c.) Salt-Water system at 40C where the salt has maximum solubility of 8.7 g salt per 100 g of water. Determine the composition of salt at the solid-liquid interface, in the liquid. Given: Mair = 29 g/mol; MH20 = 18 g/mol; Msalt = 54 g/mol
Step by Step Solution
There are 3 Steps involved in it
1 Evaluating Boundary Conditions a AirWater system at 50C with P 101325 kPa and Psat for water is 12... View full answer

Get step-by-step solutions from verified subject matter experts


