Answered step by step
Verified Expert Solution
Question
1 Approved Answer
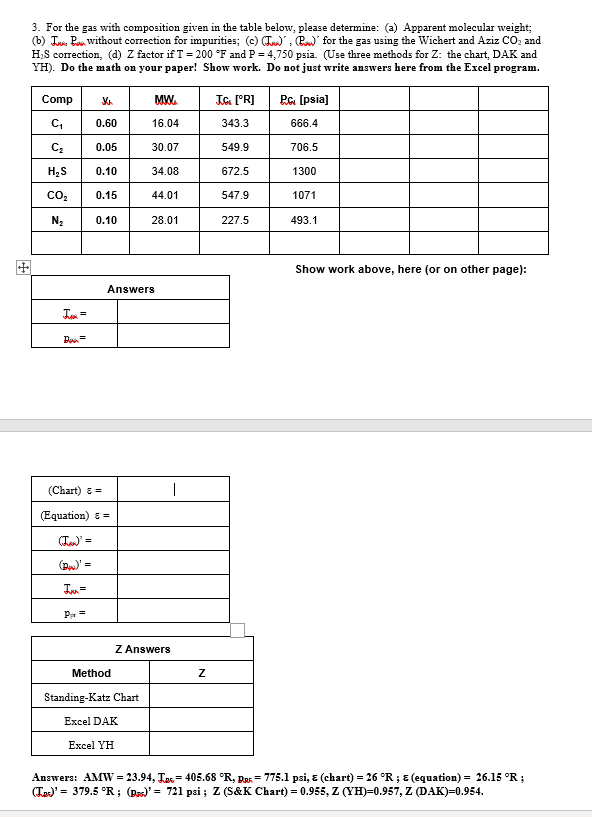
For the gas with composition given in the table below, please determine: ( a ) Apparent molecular weight; ( b ) IUwa B w without
For the gas with composition given in the table below, please determine: a Apparent molecular weight;
b IUwa without correction for impurities; cPw for the gas using the Wichert and Aziz and
correction, d factor if and psia. Use three methods for : the chart, DAK and
YH Do the math on your paper! Show work. Do not just write answers here from the Excel program.
Show work above, here or on other page:
Answers: chart;equation;
Chart
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


