Answered step by step
Verified Expert Solution
Question
1 Approved Answer
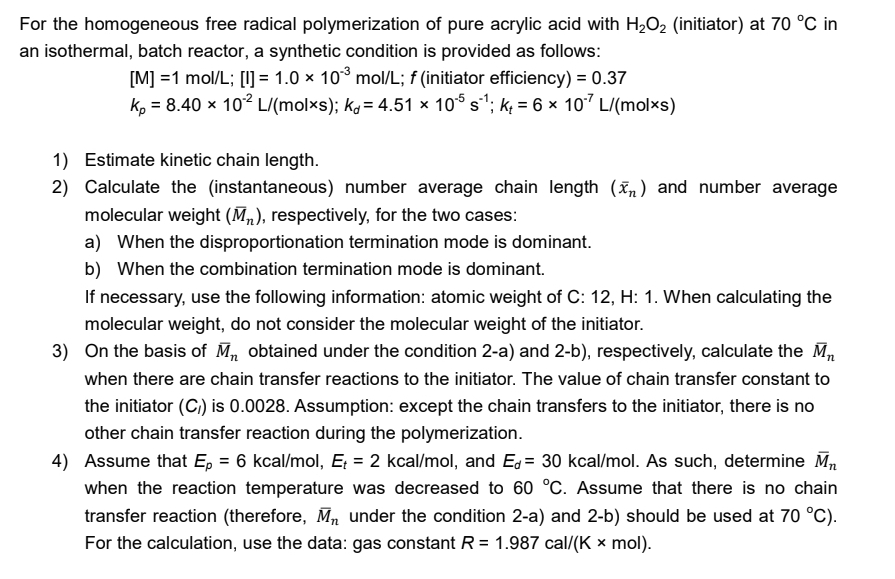
For the homogeneous free radical polymerization of pure acrylic acid with H 2 O 2 ( initiator ) at 7 0 C in an isothermal,
For the homogeneous free radical polymerization of pure acrylic acid with initiator at in an isothermal, batch reactor, a synthetic condition is provided as follows:
;; efficiency
;;
Estimate kinetic chain length.
Calculate the instantaneous number average chain length and number average molecular weight respectively, for the two cases:
a When the disproportionation termination mode is dominant.
b When the combination termination mode is dominant.
If necessary, use the following information: atomic weight of :: When calculating the molecular weight, do not consider the molecular weight of the initiator.
On the basis of obtained under the condition a and b respectively, calculate the when there are chain transfer reactions to the initiator. The value of chain transfer constant to the initiator is Assumption: except the chain transfers to the initiator, there is no other chain transfer reaction during the polymerization.
Assume that kcakca and kca As such, determine when the reaction temperature was decreased to Assume that there is no chain transfer reaction therefore under the condition a and b should be used at For the calculation, use the data: gas constant
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


