Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the honour's project, a student synthesized a poly(vinyl chloride) (PVC) by solution polymerization at 60 C as shown in the following scheme. to
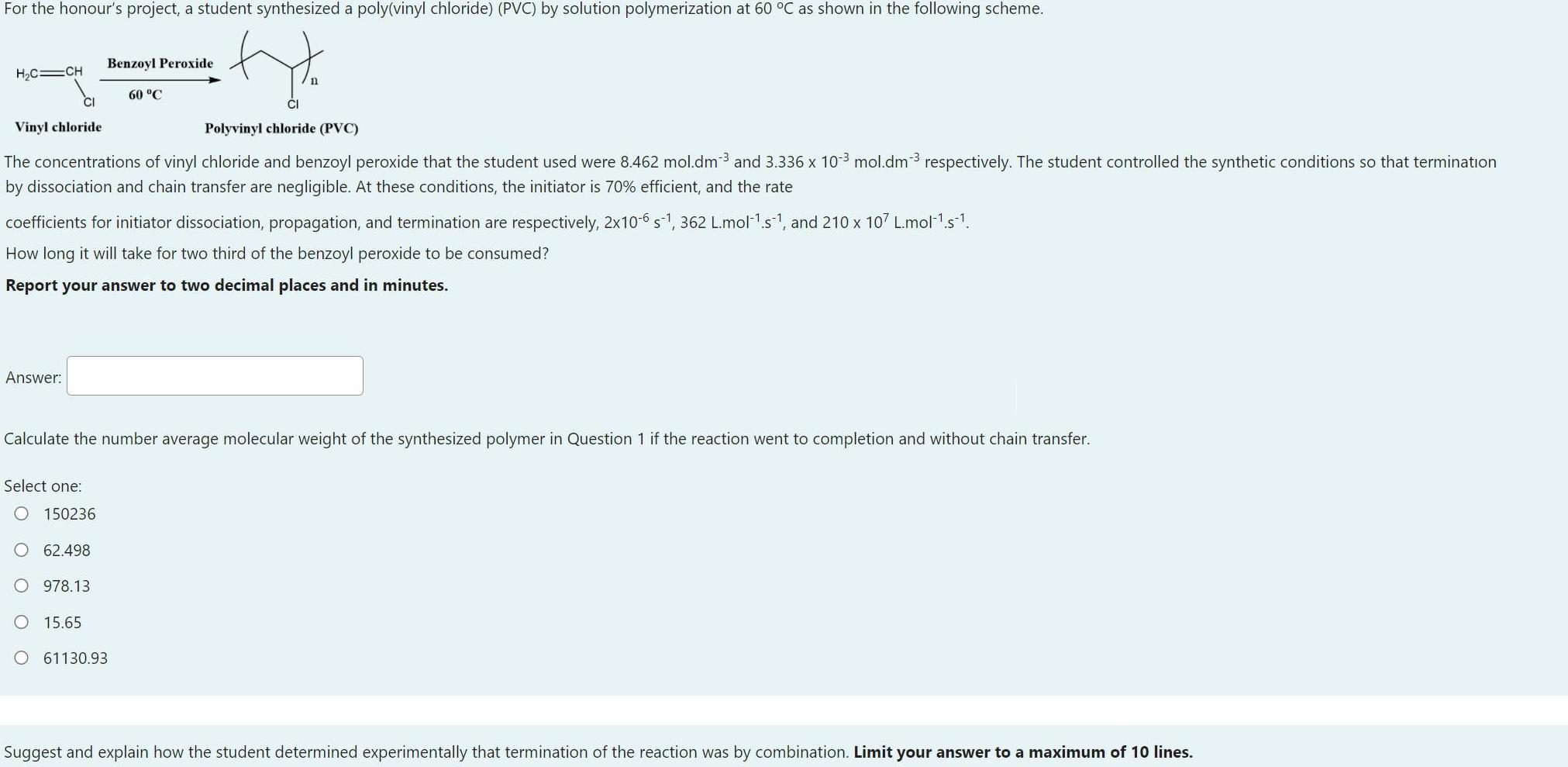
For the honour's project, a student synthesized a poly(vinyl chloride) (PVC) by solution polymerization at 60 C as shown in the following scheme. to Benzoyl Peroxide H2C CH 60 C CI Vinyl chloride Polyvinyl chloride (PVC) The concentrations of vinyl chloride and benzoyl peroxide that the student used were 8.462 mol.dm3 and 3.336 x 103 mol.dm3 respectively. The student controlled the synthetic conditions so that termination by dissociation and chain transfer are negligible. At these conditions, the initiator is 70% efficient, and the rate coefficients for initiator dissociation, propagation, and termination are respectively, 2x10-6 s-1, 362 L.mol1s1, and 210 x 107 L.molls1. How long it will take for two third of the benzoyl peroxide to be consumed? Report your answer to two decimal places and in minutes. Answer: Calculate the number average molecular weight of the synthesized polymer in Question 1 if the reaction went to completion and without chain transfer. Select one: 150236 O 62.498 O 978.13 O 15.65 O 61130.93 Suggest and explain how the student determined experimentally that termination of the reaction was by combination. Limit your answer to a maximum of 10 lines.
Step by Step Solution
★★★★★
3.29 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6361dc3c0d67b_234279.pdf
180 KBs PDF File
6361dc3c0d67b_234279.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


