Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the hot stream, we can choose any number, u feel would be better to use Figure 1 shows the process flow diagram for ethylene

For the hot stream, we can choose any number, u feel would be better to use
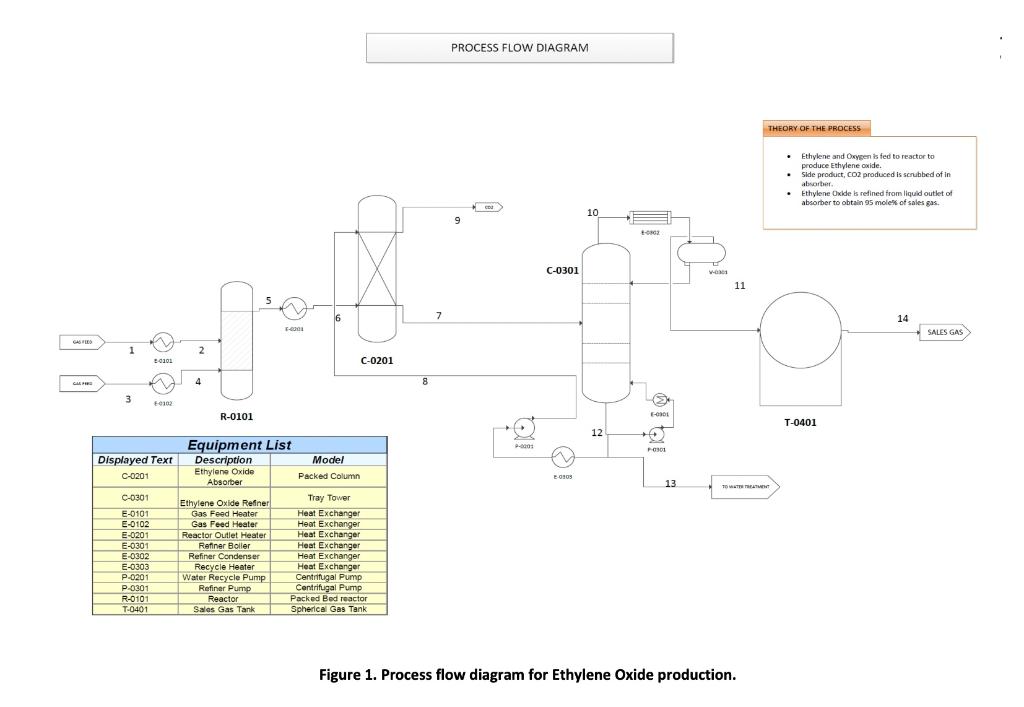
Figure 1 shows the process flow diagram for ethylene oxide (C2H4O) production. Ethylene oxide is to be synthesized from a reaction between ethylene (C2H4) and oxygen (O2) in a packed bed reactor (R0101). It has been reported that side reaction may also take place between ethylene and oxygen to produce water (H2O) and carbon dioxide (CO2) as by products. The production of ethylene oxide is described by the following stoichiometric reactions: Main reaction: C2H4+1/2O2C2H4O Side reaction: C2H4+3O22CO2+2H2O The optimum conditions for this reaction to take place is at temperature of 250C and pressure 20 bars. Thus, before entering the reactor, ethylene will be fed into a heat exchanger (E0101) with the aim to increase its temperature from 25C to 250C. Therefore, as a process engineer at the ethylene oxide production plant you are required to determine the area of the heat exchanger (E0101). For this unit, the length per pass should not be greater than 2 meters. Given in Table 1 are some of the available data. These data are very limited, and you may need to make some assumptions in your analysis. Please point out the assumptions made in calculating the heat exchanger area and show clearly all the calculations made. Identify the type of heat exchanger selected for the process, the geometric dimensions, flow pattern etc. Table 1: Available data for the processing streams Figure 1. Process flow diagram for Ethylene Oxide production. Figure 1 shows the process flow diagram for ethylene oxide (C2H4O) production. Ethylene oxide is to be synthesized from a reaction between ethylene (C2H4) and oxygen (O2) in a packed bed reactor (R0101). It has been reported that side reaction may also take place between ethylene and oxygen to produce water (H2O) and carbon dioxide (CO2) as by products. The production of ethylene oxide is described by the following stoichiometric reactions: Main reaction: C2H4+1/2O2C2H4O Side reaction: C2H4+3O22CO2+2H2O The optimum conditions for this reaction to take place is at temperature of 250C and pressure 20 bars. Thus, before entering the reactor, ethylene will be fed into a heat exchanger (E0101) with the aim to increase its temperature from 25C to 250C. Therefore, as a process engineer at the ethylene oxide production plant you are required to determine the area of the heat exchanger (E0101). For this unit, the length per pass should not be greater than 2 meters. Given in Table 1 are some of the available data. These data are very limited, and you may need to make some assumptions in your analysis. Please point out the assumptions made in calculating the heat exchanger area and show clearly all the calculations made. Identify the type of heat exchanger selected for the process, the geometric dimensions, flow pattern etc. Table 1: Available data for the processing streams Figure 1. Process flow diagram for Ethylene Oxide productionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


