Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For the reaction: H(g)+Cl(g)HCl(g), What is the expression of the equilibrium constant Kp in terms of molecular properties at 2000K ? You don't need to
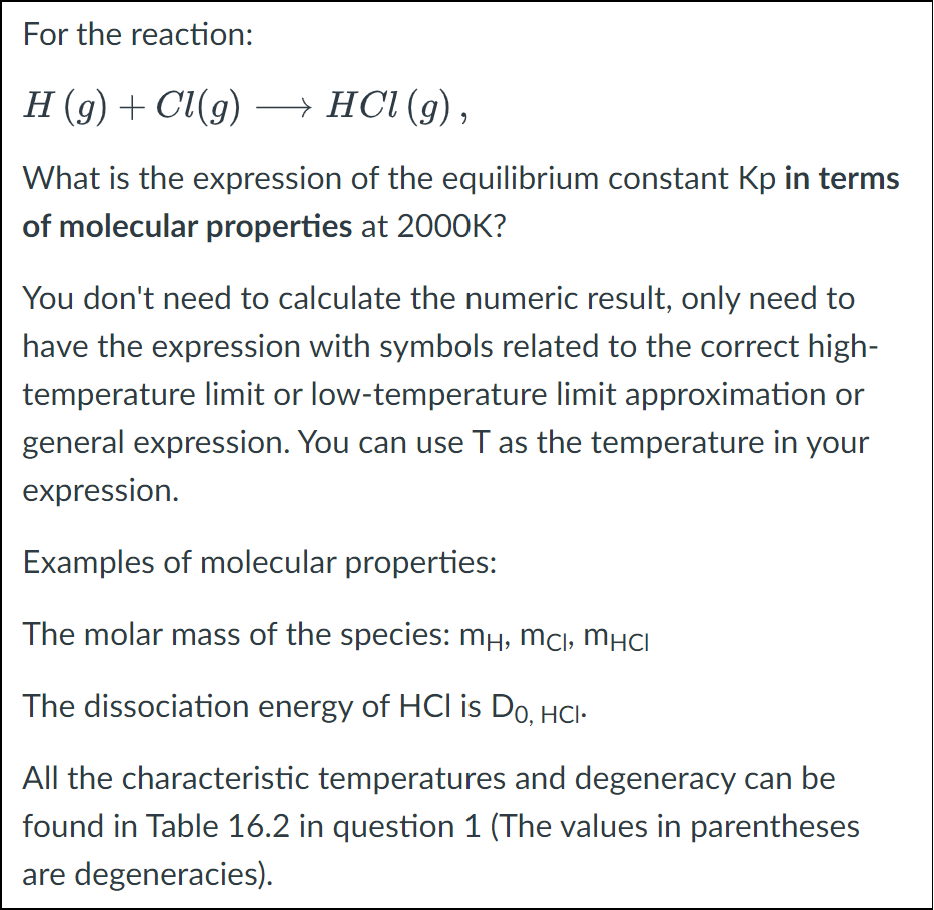 For the reaction: H(g)+Cl(g)HCl(g), What is the expression of the equilibrium constant Kp in terms of molecular properties at 2000K ? You don't need to calculate the numeric result, only need to have the expression with symbols related to the correct hightemperature limit or low-temperature limit approximation or general expression. You can use T as the temperature in your expression. Examples of molecular properties: The molar mass of the species: mH,mCl,mHCl The dissociation energy of HCl is D0,HCl. All the characteristic temperatures and degeneracy can be found in Table 16.2 in question 1 (The values in parentheses are degeneracies)
For the reaction: H(g)+Cl(g)HCl(g), What is the expression of the equilibrium constant Kp in terms of molecular properties at 2000K ? You don't need to calculate the numeric result, only need to have the expression with symbols related to the correct hightemperature limit or low-temperature limit approximation or general expression. You can use T as the temperature in your expression. Examples of molecular properties: The molar mass of the species: mH,mCl,mHCl The dissociation energy of HCl is D0,HCl. All the characteristic temperatures and degeneracy can be found in Table 16.2 in question 1 (The values in parentheses are degeneracies) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


