Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) What is the rate law for this reaction? b) What is the rate constant for this reaction? Don't forget units! )A two-step mechanism

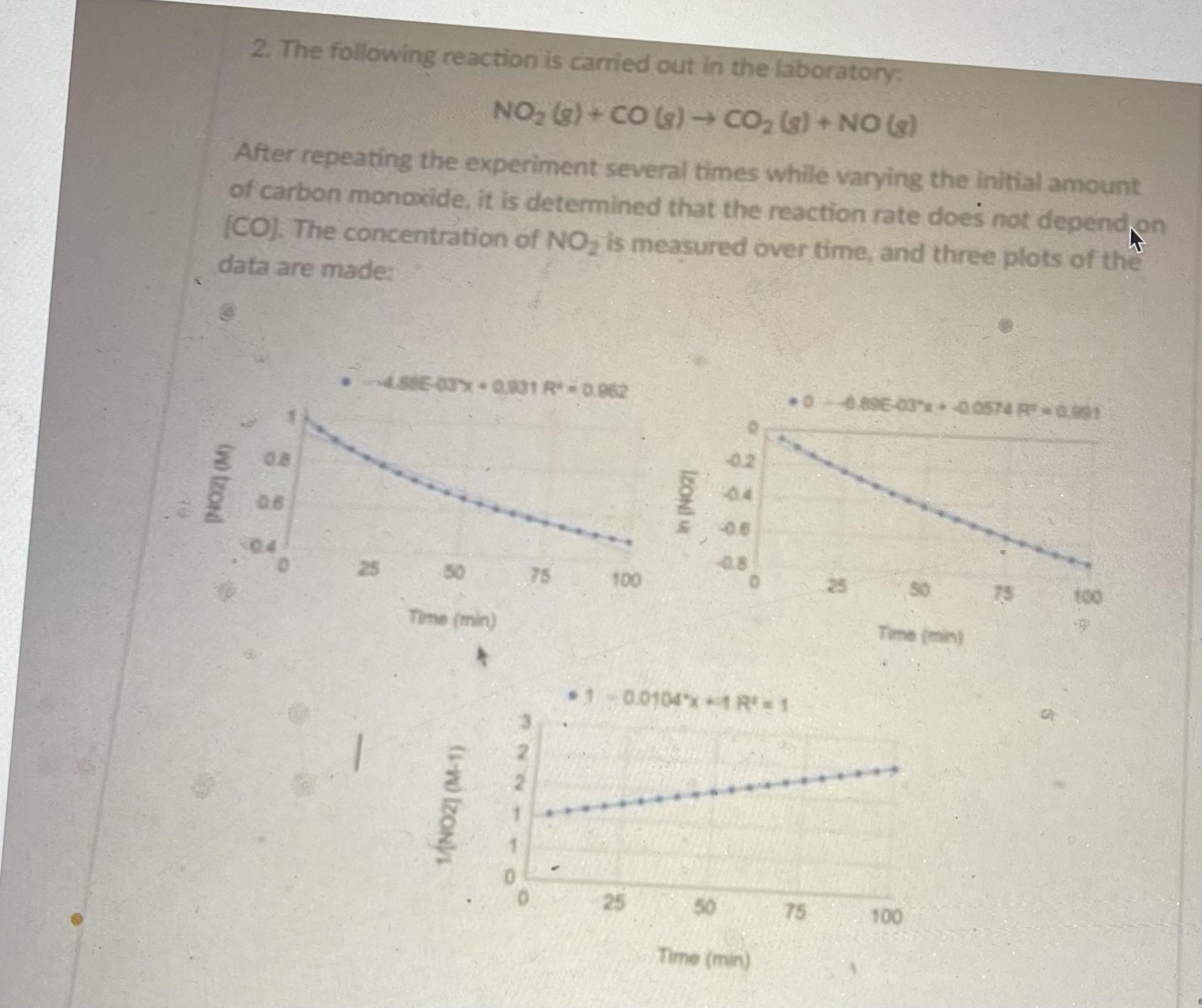
a) What is the rate law for this reaction? b) What is the rate constant for this reaction? Don't forget units! )A two-step mechanism is proposed for this reaction. Write the chemical equation for the second step. Step 1:2 NO2 (g) - NO, (3) + NO (3) Step 2: ?? d) Which step has the larger activation barrier, step 1 or step 2? Explain your answer in 1-2 sentences. 2. The following reaction is carried out in the laboratory NO2 G) + CO (s) CO, (s)+ NO (e) After repeating the experiment several times while varying the initial amount of carbon monaxide, it is determined that the reaction rate does not depend on [CO]. The concentration of NO, is measured over time, and three plots of the data are made: 4.88E-03x 0831 R0.962 06896-03* 0.0574 R 0.991 02 0.8 04 08 0.6 04 08 25 50 75 100 25 50 75 100 Time (min) Time (min) 0.0104x1 R= 1 25 50 75 100 Time (min) IZONI M 1NOZI (M-1) ino tzond
Step by Step Solution
★★★★★
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


