Question
For water (w) and H2O2 (hp) solutions at 333.15 K, some liquid and vapor compositions and (total) vapor pressures are given in the table below.
For water (w) and H2O2 (hp) solutions at 333.15 K, some liquid and vapor compositions and (total) vapor pressures are given in the table below.
Pure liquid vapor pressures at 333.15 K are Pw* 19.92 kPa and Php* 2.35 kPa. For the solution xwl 0.300 at 333.15 K:
(a) Calculate γI and aI for water and H2O2.
(b) If the solvent is taken as water, calculate γII and aII for water and H2O2. (Note: you need to find Henry's constant, K)
(c) Calculate the ΔG-mixture for this solution when 125 g of solution is formed from the pure components at this mole fraction and constant T and P^.
(d) Calculate the excess free gibbs energy (ΔGF) for this solution.
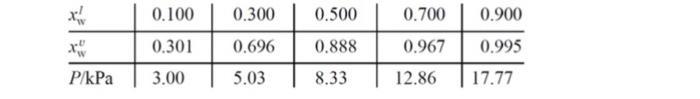
0.100 0.300 0.500 0.700 0.900 0.301 0.696 0.888 0.967 0.995 P/kPa 3.00 5.03 8.33 12.86 17.77
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



