Answered step by step
Verified Expert Solution
Question
1 Approved Answer
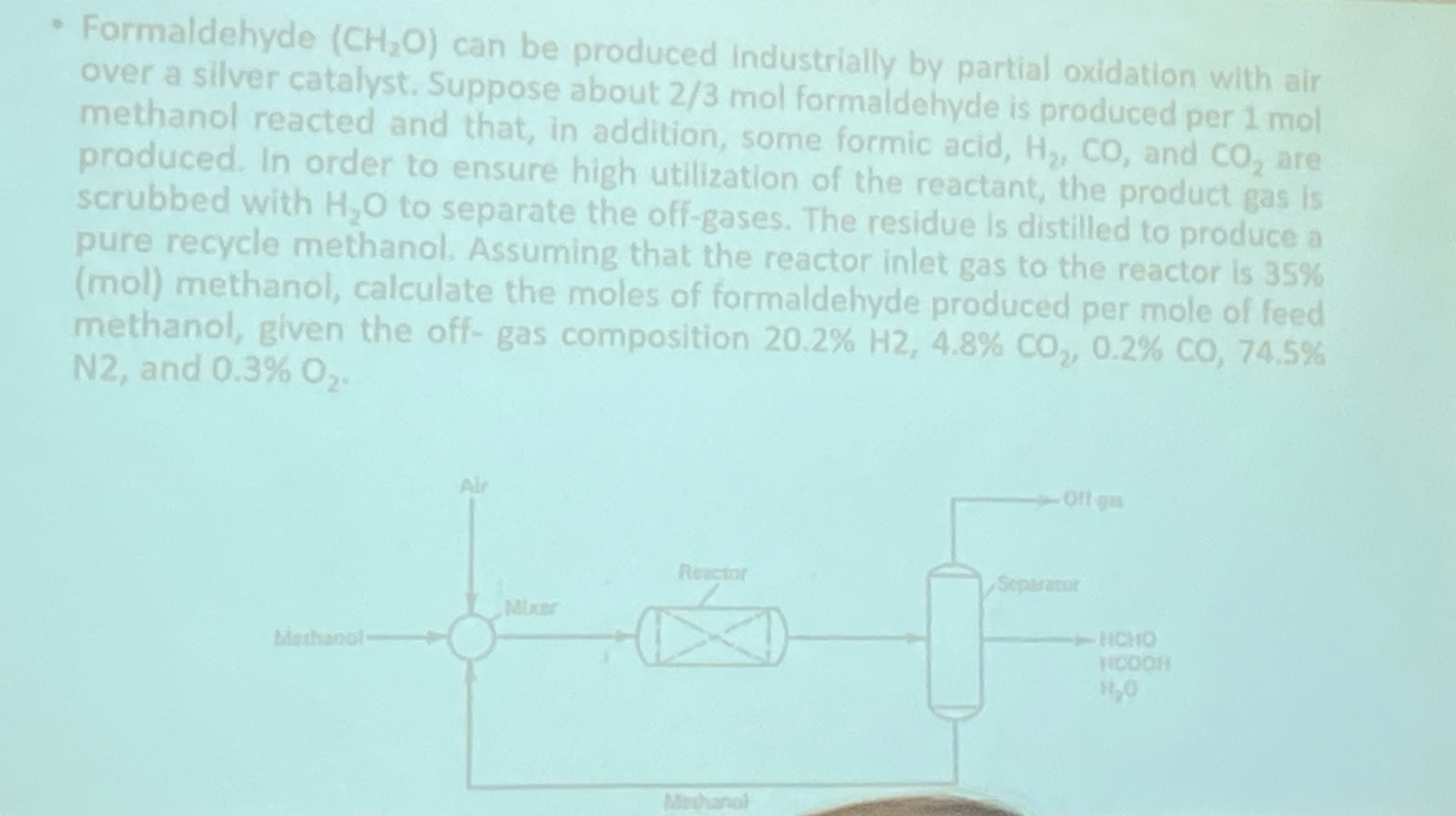
Formaldehyde ( C H 2 O ) can be produced industrially by partial oxidation with air over a silver catalyst. Suppose about 2 3 mol
Formaldehyde can be produced industrially by partial oxidation with air over a silver catalyst. Suppose about mol farmeldahyde ts produced per mol methanol reacted and that, in addition, some formic acid, and are produced. In order to ensure high utilization of the reactant, the product gas is scrubbed with to separate the offgases. The residue Is distilled to produce a pure recycle methanol. Assuming that the reactor inlet gas to the reactor is mol methanol, calculate the moles of formaldehyre nrodiced ner mot of methanol, given the offgas composition and
Use mass balances to solve
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


