Question
Formaldehyde is a common preserative agent used in a wide variety of products ranging from pressed wood, cosmetics, embalming fluids and vaccines to list
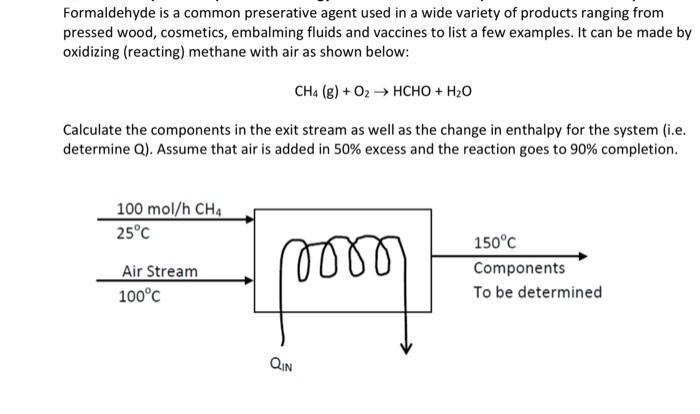
Formaldehyde is a common preserative agent used in a wide variety of products ranging from pressed wood, cosmetics, embalming fluids and vaccines to list a few examples. It can be made by oxidizing (reacting) methane with air as shown below: CH4 (g) + O2 HCHO + H20 Calculate the components in the exit stream as well as the change in enthalpy for the system (i.e. determine Q). Assume that air is added in 50% excess and the reaction goes to 90% completion. 100 mol/h CH4 25C 150C Air Stream Components 100C To be determined QIN
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
100 malho CHY 15c Ais stream looc Qin CHA 02 HCHO H2O Here since no moes ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials of Materials Science and Engineering
Authors: Donald R. Askeland, Wendelin J. Wright
3rd edition
978-1111576868, 1111576866, 978-1285677620, 1285677625, 978-1111576851
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



